Hello friends! If you are a student of science, it is very important for you to know about the concept of valency. So today we shall discuss Carbon Valence Electrons. Valency is the measure of the potential of the atom of an element to form a bond with the atom of another element to reach the octet or the inert gas stage.
- Flerovium Valence Electrons
- Mercury Valence electrons
- Moscovium Valence Electrons
- Bismuth Valence electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Oganesson Valence Electrons
- Tellurium Valence Electrons
- Nobelium Valence Electrons
- Xenon Valence Electrons
- Neptunium Valence Electrons
- Cesium valence electrons
- Plutonium Valence Electrons
- Iodine Valence Electrons
- Americium Valence Electrons
- Radium Valence Electrons
- Gold Valence electrons
- Lead Valence electrons
- Boron Valence Electrons
- Nitrogen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- Aluminum Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Argon Valence Electrons
- Calcium Valence Electrons
How many valence electrons does carbon have?
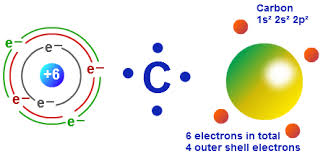
Since the Carbon atom has four electrons in its outer shell and needs four more electrons to complete its octet, its valency is 4.
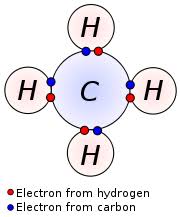
Carbon Valence Electrons Dot Diagram
Valency of Carbon
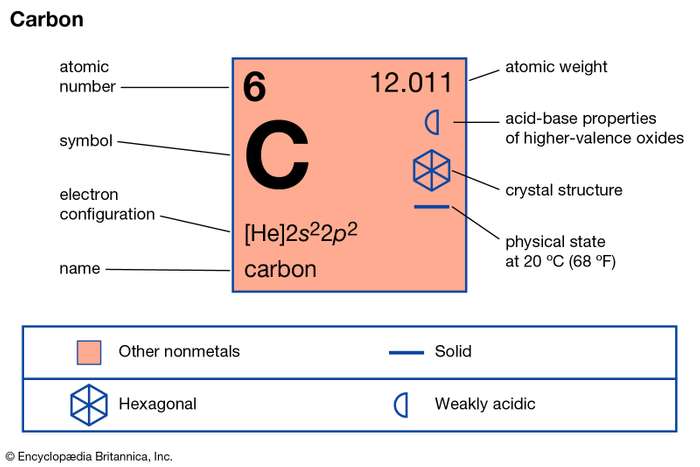
We can find the valency of the carbon atom by looking at which group it belongs in the periodic table. Since it belongs to group 4, its valency is 4. Also, since its atomic number is equal to four, its number of valence electrons is equal to the group it belongs in the periodic table.
Valence Electrons in a Carbon Atom in the Ground State
Carbon has 6 electrons in its ground or neutral state. Hence it has 2 electrons in its first shell and 4 electrons in its second or outer shell. Hence it has four valence electrons. The Valence Electrons of Many elements have been provided in this website. Check the Iron Valence Electrons
Leave a Reply