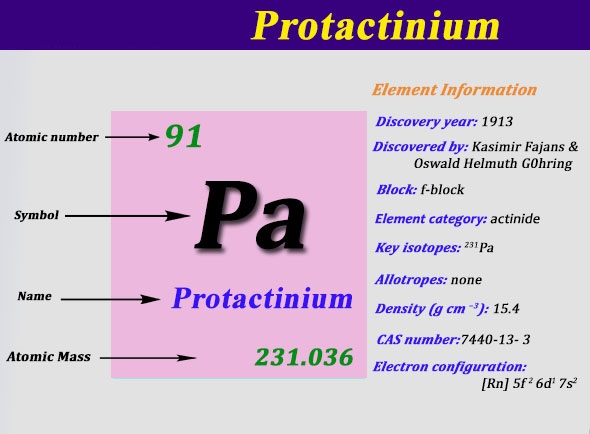
Protactinium Electron Configuration: Protactinium (formerly called protoactinium) is a chemical element that has a chemical symbol Pa. The atomic number of Protactinium is 91. It is a silvery-grey, dense, actinide metal that readily reacts with water vapour, oxygen, and inorganic acids.
- Hydrogen Electron Configuration
- Silicon Electron Configuration
- Helium Electron Configuration
- Lithium Electron Configuration
- Aluminum Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Scandium Electron Configuration
- Sulfur Electron Configuration
- Sodium Electron Configuration
- Germanium Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
It is found in typically few parts per trillion in the Earth’ s crust but may also reach to a few parts per million in some uraninite ore deposits. Because of its high radioactivity, high toxicity and scarcity, there is no current use for protactinium except scientific research, and for this reason, it is mostly extracted from spent nuclear fuel.
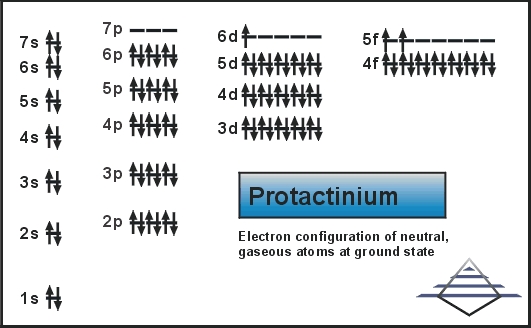
Protactinium Electron Configuration
It was first identified by Oswald Helmuth Göhring and Kasimir Fajans in 1913 and named premium because of the short half-life of the specific isotope studied, that is protactinium-234. 231Pa, the more stable isotope of protactinium was discovered by Otto Hahn and Lise Meitner 231Pa, and they chose the name proto-actinium, but the IUPAC in 1949finally named it “protactinium” and confirmed Meitner and Hahn as discoverers.
Today we will share the information about the electron configuration of the Protactinium. Please see the full post below.
What is the Electron Configuration of Protactinium
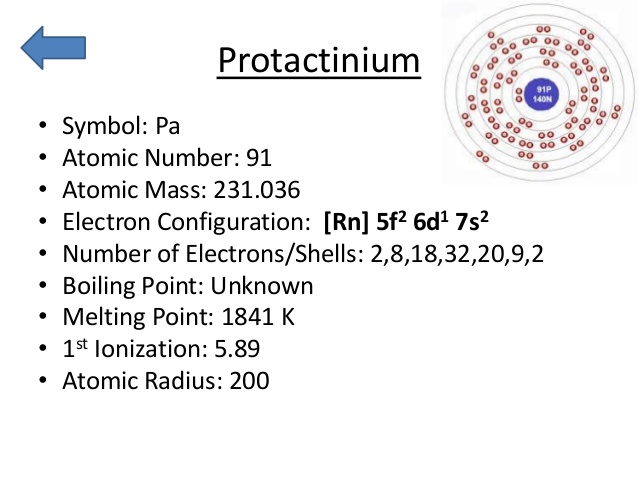
Rn 5f2 6d1 7s2 is the electron configuration of the protactinium.
How Many Valence Electrons Does Protactinium have
There are five valence electrons in the last shell of the protactinium.
Protactinium Number of Valence Electrons
Protactinium has five valence electrons in its outer shell.