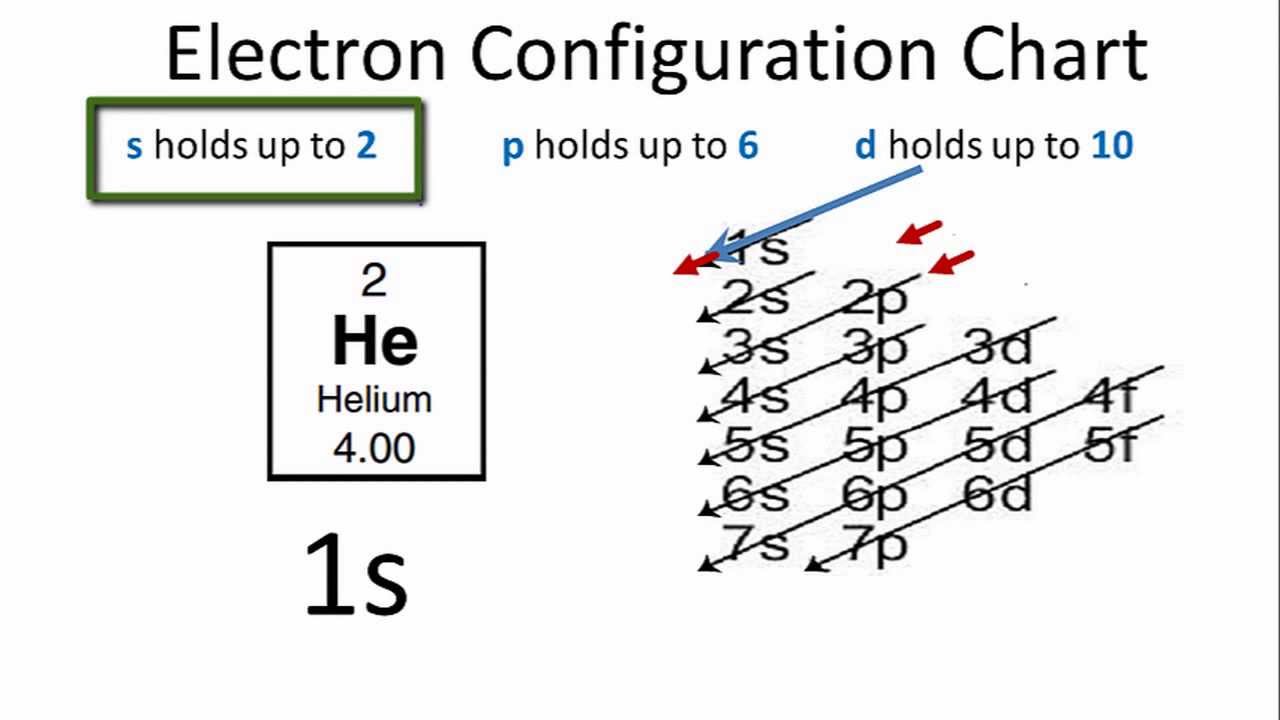
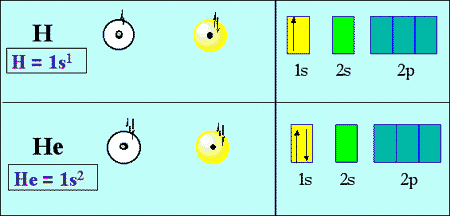
Helium Electron Configuration: In the periodic table, the second chemical element is Helium whose symbol is “He” and its atomic number is 2. Helium is the chemical element that is tasteless, colorless, odorless, inert, non-toxic, monoatomic gas and it lies in the noble gas group category. This element has the lowest boiling point.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
As the electrons distribution on the orbit of a particular atom/molecule is defined as the electron configuration of that atom or molecule. Similarly, helium has two electrons present at its first shell or orbit so the electronic configuration of helium is represented as:
Helium Electronic Configuration
1s2
Electron Configuration For Helium Ion
Helium has two electrons which are present at its first orbit that is 1s. So, the electron configuration for helium ion is 1s2.
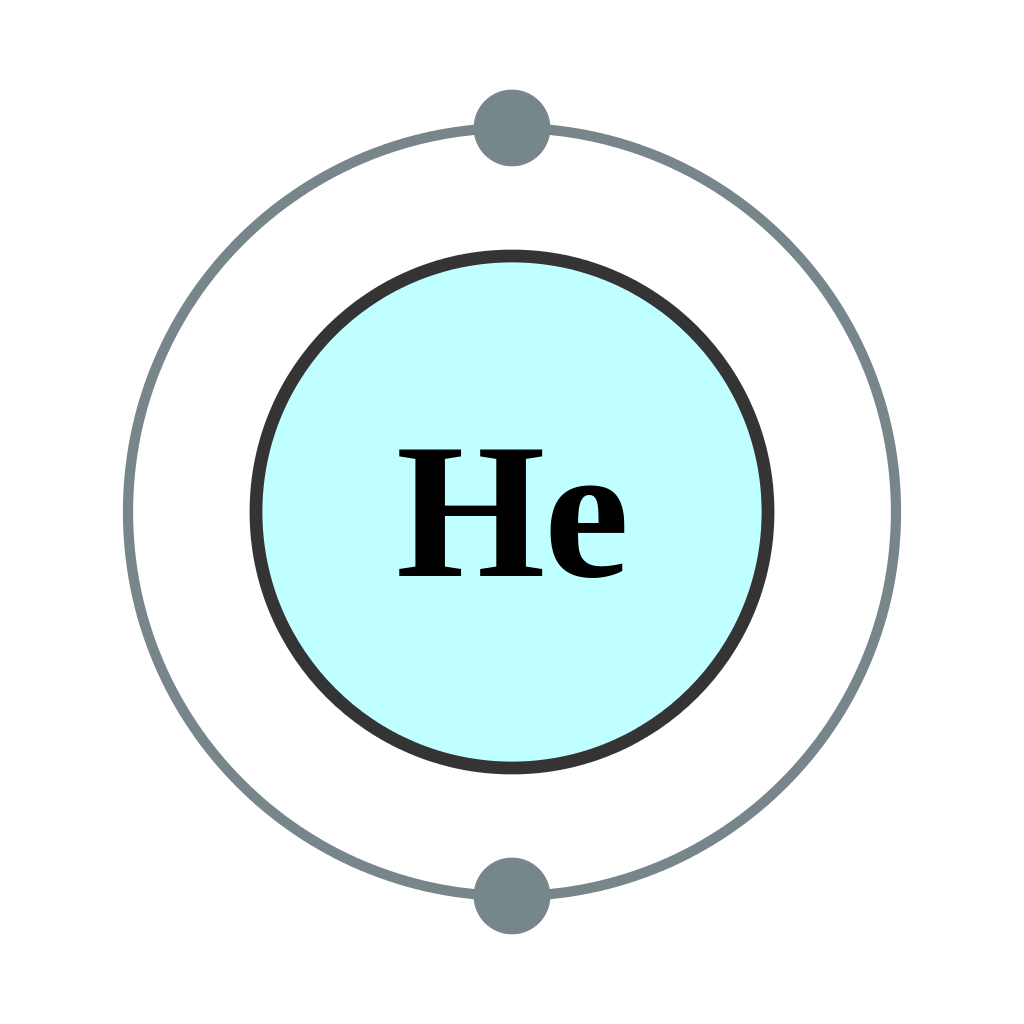
Helium Number of Valence Electrons
Valence electrons are the number of electrons present at the outermost shell. Here the helium has only two electrons which are present in its one shell. So, the number of valence electrons for Helium is 2.
What Is The Electron Configuration of Helium
The electron configuration of this noble gas element is 1s2 as He consists of 2 electrons.
How Many Valence Electrons are in helium
Helium has 2 valence electrons as the numbers of electrons which are in the outer orbit are considered as valence electrons and helium with 2 electrons is stable and the number of valence electrons is also 2 here.