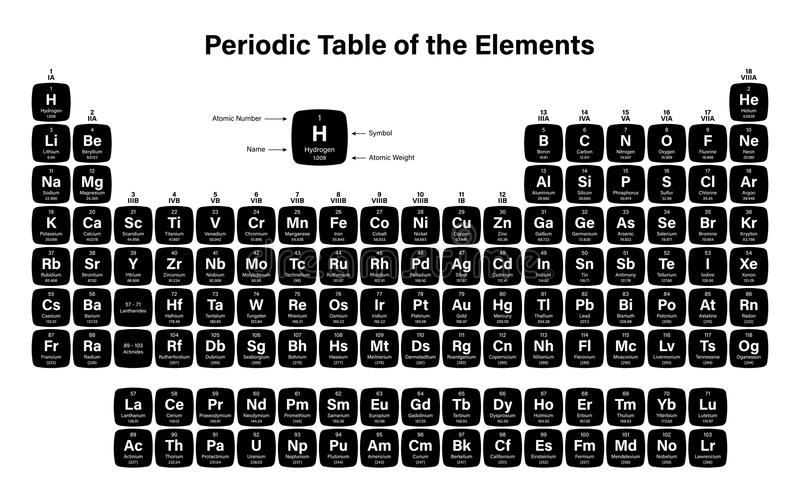
The periodic table of elements is the table that arranges all the chemical elements in order of their increasing atomic number. This periodic table of elements was given by Dmriti Mendeleev.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
Atomic Mass of First 30 Elements
First thirty elements of periodic table are Hydrogen, helium, lithium, beryllium, Boron, carbon, nitrogen, oxygen, fluorine, Neon, sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, argon, potassium, calcium, scandium, titanium, vanadium, chromium, manganese, iron, Nickel, cobalt, copper, zinc. We are providing the periodic table that includes atomic masses of all these thirty elements.
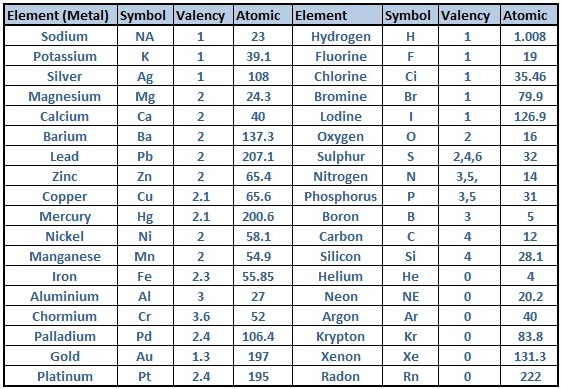
Periodic Table of Elements With Atomic Mass And Valency
Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical. We are providing the periodic table that contains both valency and atomic mass of the elements. You can check that out below;
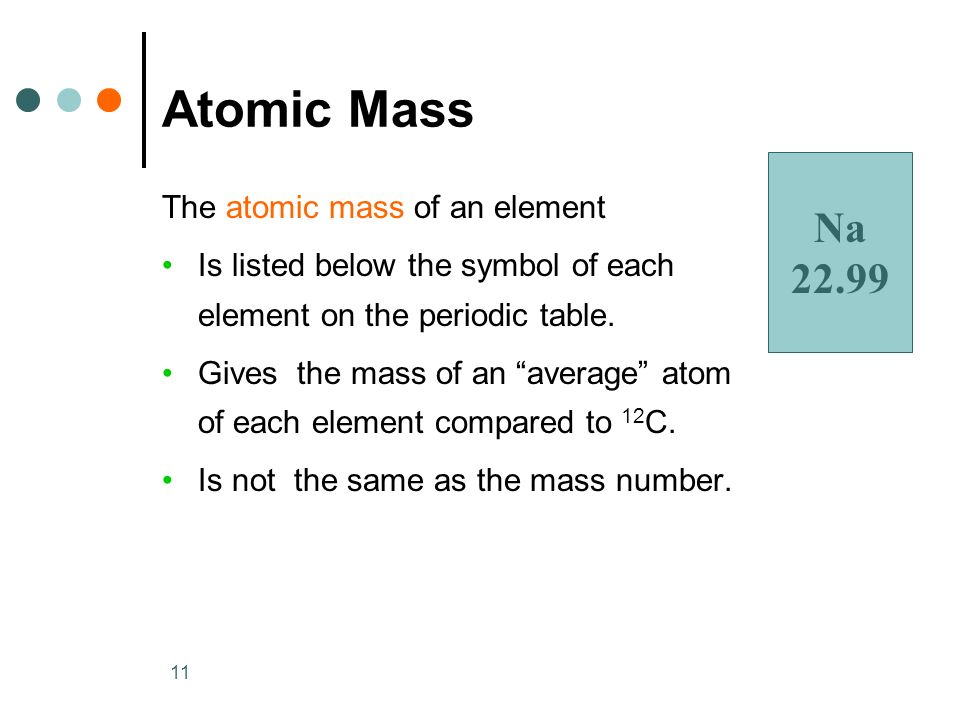
What is The Atomic Mass of An Element?
Atomic mass is the measure of the mass of atom of an element.
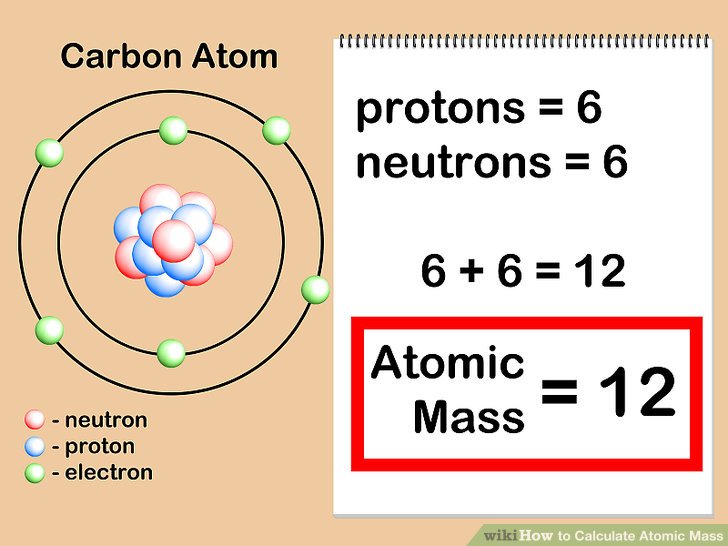
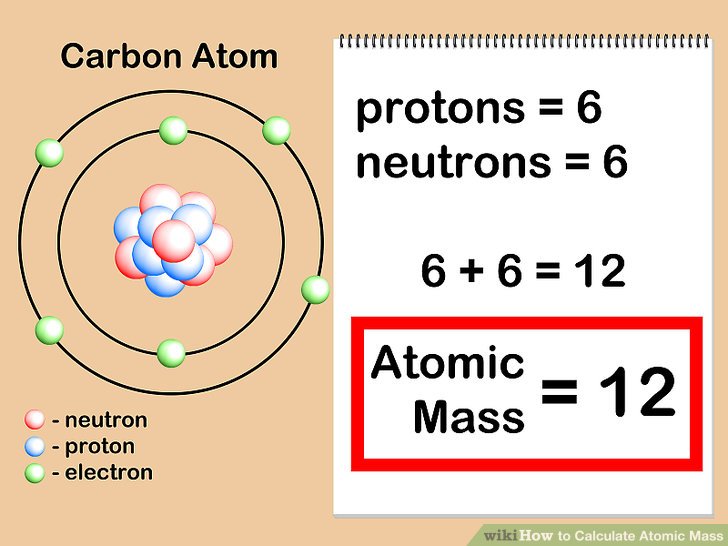
How To Find Atomic Mass of Elements
Atomic mass of an element is calculated by adding the mass of neutron and proton of atom of an element.
How Do You Calculate Atomic Mass?
The formula for calculating atomic mass is given in the picture below;
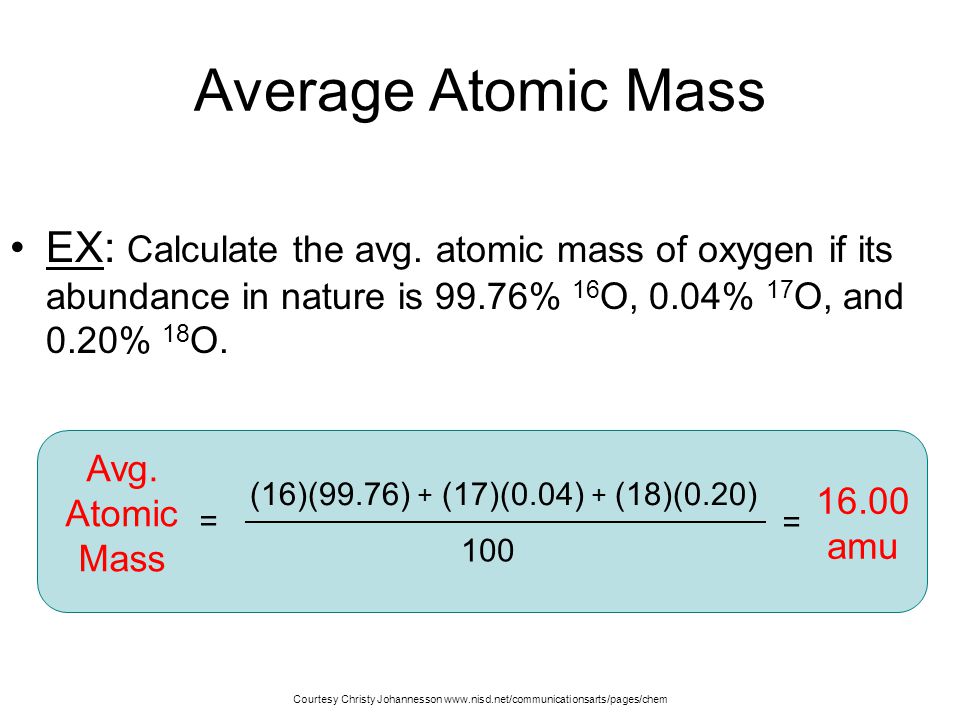
Average Atomic Mass
Periodic Table With Names And Atomic Mass And Number
It becomes to learn and to study when you have all the study material with you. Here we are providing the periodic table that contains the name, atomic mass and atomic number of an element.
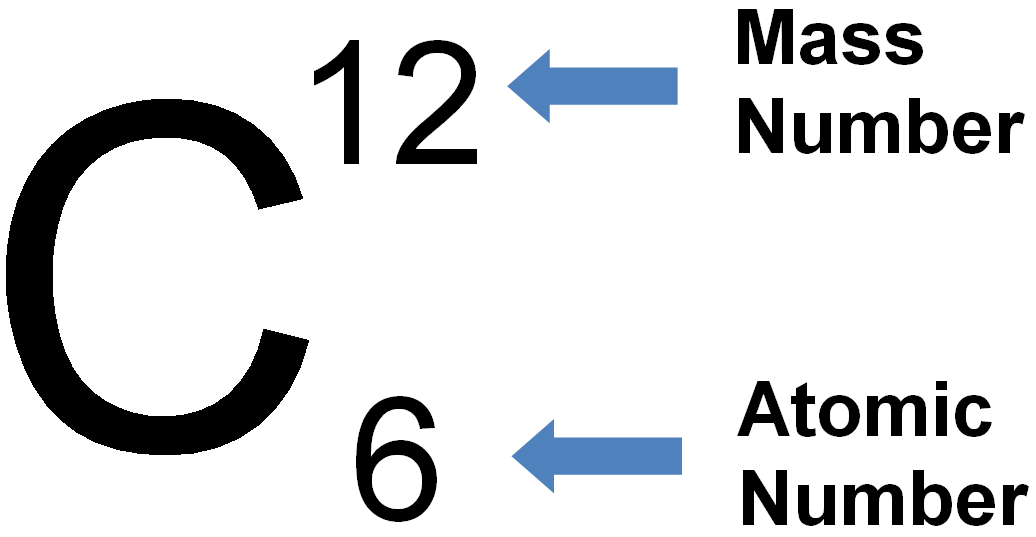
What is The Atomic Mass Number?
Atomic mass number is also called nucleon number. It is the total number of neutron and proton in an atomic nucleus. The element arranged in the periodic table also contains the mass number.
How Do You Calculate The Mass?
Mass is calculated by multiplying the volume and density of the element.

Leave a Reply