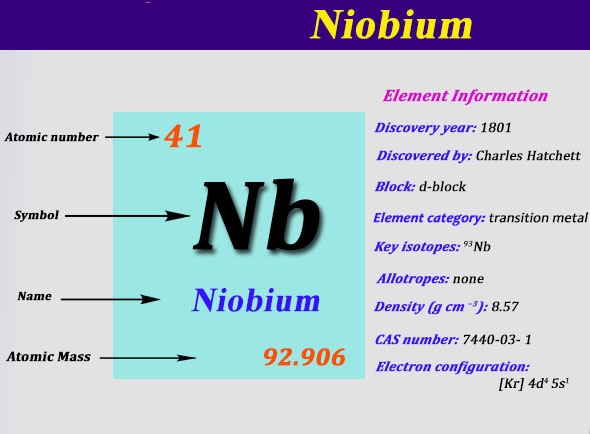
Niobium Electron Configuration: Niobium is commonly known as Columbium. It is a chemical element that has a symbol Nb. The atomic number of Niobium is 41. It is a grey, soft, ductile, crystalline, transition metal, that is often found in the minerals columbite and pyrochlore. Niobium was officially recognized as the element in 1949, but the name columbium is currently used in metallurgy in the United States.
- Bromine Electron Configuration
- Sodium Electron Configuration
- Magnesium Electron Configuration
- Aluminum Electron Configuration
- Silicon Electron Configuration
- Sulfur Electron Configuration
- Chlorine Electron Configuration
- Phosphorus Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
- Scandium Electron Configuration
Niobium Electron Configuration
Charles Hatchett, the English chemist reported a similar new element in 1801 and named it columbium. In 1809, William Hyde Wollaston, the English chemist concluded wrongly that columbium and tantalum were identical.
Heinrich Rose, the German chemist, in 1846 determined that tantalum ores contain another element, which he called niobium. In the years 1864 and 1865, some of the scientific observations clarified that columbium and niobium were the same elements and for about a hundred years both names were used. Niobium was officially recognized as the name of the element in 1949. Today we are going to share the
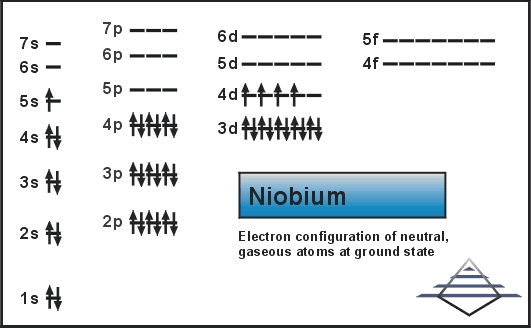
What is the Electron Configuration of Nb
Kr 4d4 5s1 is the electron configuration of Niobium.
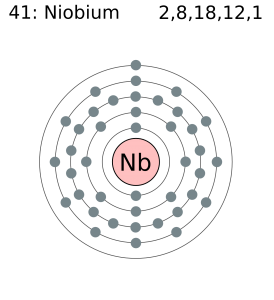
How Many Valence Electrons Does Niobium Have
There are 41 valence electrons in the outer shell of Niobium.
Niobium Number of Valence Electrons
Niobium has 41 valence electrons in its outer shell.
Leave a Reply