Indium Electron Configuration: Indium is a chemical element that has a chemical symbol In and atomic number 49. It is a post-transition which makes up to 0.21 parts per million of the Earth surface. It is malleable and very soft and has a melting point higher than gallium and sodium, but it is lower than lithium and tin. Chemically, indium is similar to thallium and gallium.
- Sodium Electron Configuration
- Germanium Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
Electron Configuration For Indium
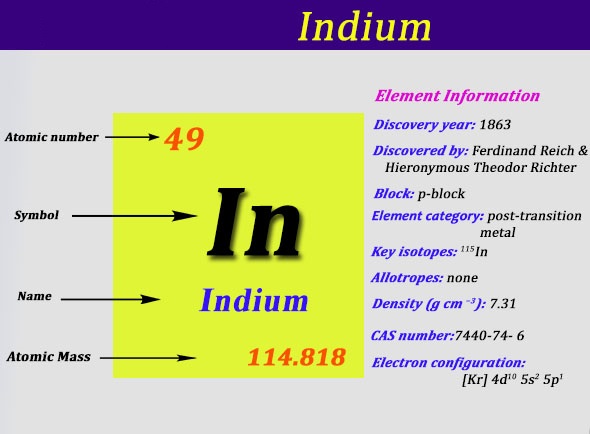
It is largely intermediate between the two in terms of its characteristics. It was discovered by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods in 1863. They name it because of the indigo blue line in its spectrum. It is a smaller component in zinc sulfide ores and is produced and a byproduct of zinc refinement.
It is used in the semiconductor industry, at low-melting-point metal alloys like soldiers, and also used in the production of transparent conductive coatings of indium tin oxide on glass. Today we are going to provide you with all details about the electron configuration of In.
What is the Electron Configuration of Indium
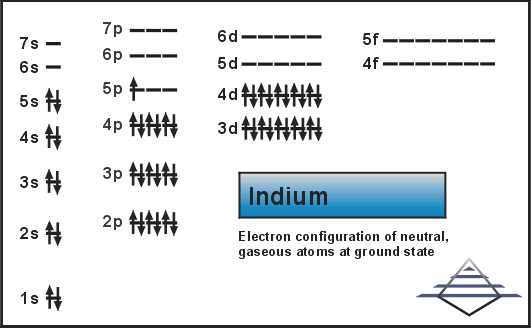
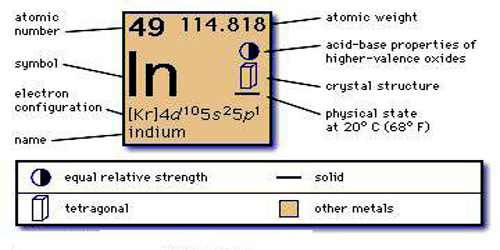
Kr 4d10 5s2 5p1 is the electron configuration of In.
How Many Valence Electrons Does Indium Have
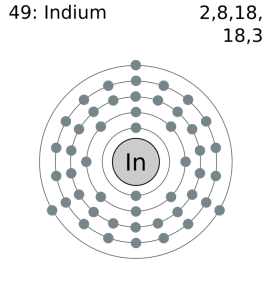
Indium has three valence electron in its outer shell.
Indium Number of Valence Electrons
There are three valence electrons in the outer shell of In.
Ground State Electron Configuration For Indium
[Kr].4d10.5s2.5p is the ground state electron configuration for In.
Leave a Reply