Hello friends! We shall discuss Oxygen Valence Electrons. As you know, valency is the potential of an atom to form a bond with the atom of another element. It is especially the measure of the number of Hydrogen atoms an element can displace or form the bond with.
- Flerovium Valence Electrons
- Mercury Valence electrons
- Moscovium Valence Electrons
- Bismuth Valence electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Oganesson Valence Electrons
- Tellurium Valence Electrons
- Nobelium Valence Electrons
- Xenon Valence Electrons
- Neptunium Valence Electrons
- Cesium valence electrons
- Plutonium Valence Electrons
- Iodine Valence Electrons
- Americium Valence Electrons
- Radium Valence Electrons
- Gold Valence electrons
- Lead Valence electrons
For Blank Periodic Table And Periodic Table Valency Of Elements.
Oxygen Valence Electrons
Oxygen has 8 valence electrons. Hence it belongs to group 6 of the periodic table. This means it has a total of 2 electrons in the first shell (or K shell), and 6 electrons in the first shell (or K shell). Hence it has a valency of 6.
Latest Periodic Table Of Lebeled And Periodic Table Atomic Mass Number.
What is the Valence of Oxygen in Water
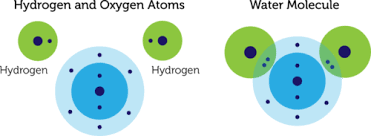
When an oxygen atom forms a covalent bond with two Hydrogen atoms, it forms water (or H2O.) An oxygen atom contains 8 electrons that revolve around two orbital shells. The first shell, or the inner shell, contains 2 electrons. Whereas, the outer shell contains 6 electrons.
How many valence electrons does oxygen have?
Click Here To More Information About Periodic Table Charges Of Elements And Periodic Table Of Trends.
Hydrogen has only one electron which revolves around the proton in a single orbital. Hence both the atoms of the elements are unstable.
To achieve an inert gas electronic configuration or an octet, the oxygen atom must contain 2 more electrons in its outer shell. So to reach this stage, it forms a covalent bond with two Hydrogen atoms to form a covalent bond.
Hence the valency of oxygen in water is 2 since it shares two electrons with Hydrogen to reach the inert gas octet electronic configuration.
Find Our Periodic Table Of Non-Metals And Electronegativity Chart.
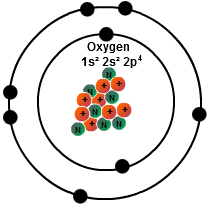
There are only 6 valence electrons. Valence electrons are the ones in the outermost shell. The shell has a capacity of 8 which is the maximum for that shell. The fact that an additional 2 electrons could exist in the second shell/ring is how the oxygen atom can bond to other atoms through ionic bonding. The maximum number of electrons which could fill a specific shell is found with the general formula 2(n*n) where n = the shell number. The 3rd shell could have a maximum of 2(3*3) = 2*9 = 18 electrons. The 4th shell …. 2(4*4) = 2*16 = 32. Also the sum of the number of electrons in all of the shells of and atom is equal to the number of protons. This gives the atom a net charge of zero. Gaining or losing an electron(s) results in a positive (+) or negative (-) ion.
This means it has a total of 2 electrons in the first shell (or K shell), and 6 electrons in the first shell (or K shell)
??