Nickel Electron Configuration: Nickel is a chemical element which has a 28th position in the periodic table. The symbol of Nickel is “Ni” and its atomic number is 28. Nickel is a silvery-white lustrous metal which has a golden tinge. This metal is hard and ductile. It belongs to a family of elements which are “transition metals”.
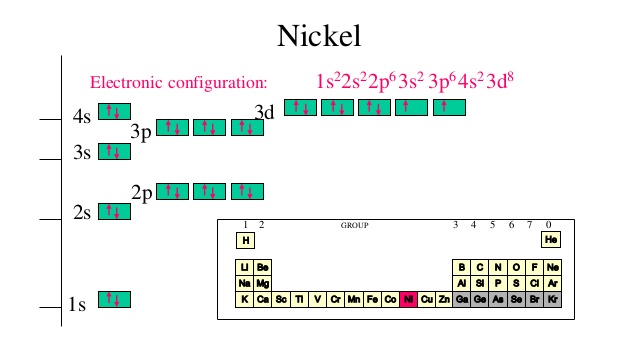
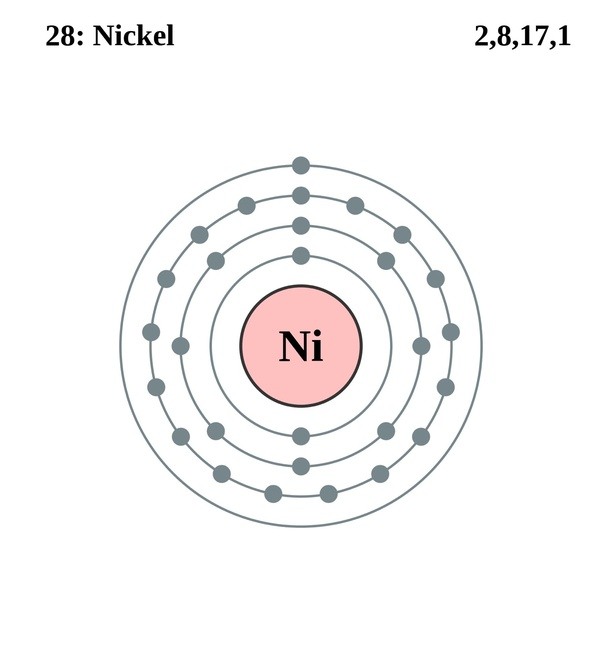
The electronic configuration is defined as the number of electrons distributed in the atom’s or molecule’s orbits. Nickel has 28 electrons which are distributed among the 4 orbits of its atom.
Electronic configuration of nickel can be written as: 1s22s22p63s23p63d84s2 or it can also be written as [Ar] 3d84s2
What is The Electron Configuration of Nickel
The nickel consists of 28 electrons in the atomic shell in 4 orbits. Electron configuration is the distribution of electrons in the orbits of atoms or molecules.
The electronic configuration of nickel is: 1s22s22p63s23p63d84s2 or you can also write it as [Ar] 3d84s2
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Technetium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
How Many Valence Electrons Does Nickel Have
The electrons located in the outermost shell are considered as valence electrons. In the case of nickel, there are 2 electrons present in the 4th or outermost shell. So, valence electrons of nickel are 2.
Nickel Number of Valence Electrons
Valence electrons are the electrons which are located in the outer shell or orbit. There are 28 electrons in the nickel in the 4 orbits and the number of electrons present in the outermost shell is 2. That is, nickel consists of 2 valence electrons.