Check out the Xenon electron configuration here and get the proper understanding of this chemical element for yourself. The article contains the proper information on the chemical properties and the electron configuration for ease of understanding to the readers.
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
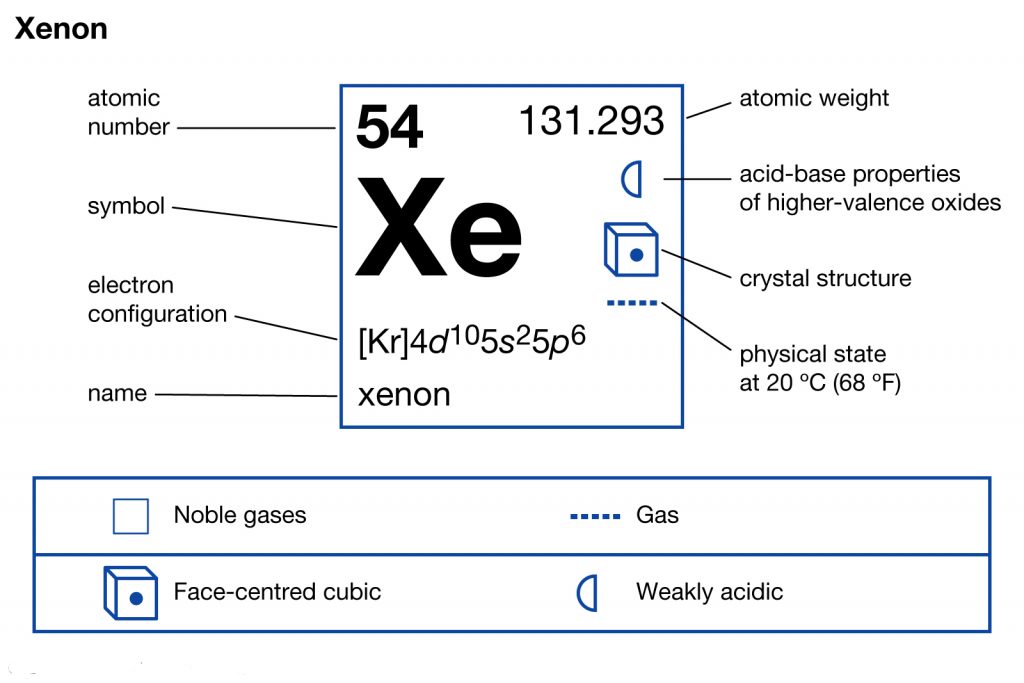
Xenon is a well-known chemical element in the chemistry domain of science. It has the atomic number 54 and the representative sign as Xe. Xenon is precisely the gas that has the texture as the colorless, dense, and odorless liquid. You can easily find this chemical element in the atmosphere of earth with a significant amount.
Xenon Electron Configuration
Xenon falls in the category of noble gas and there are some other naturally occurring forms of this gas. For instance, you can also find it as the component of some naturally occurring mineral spring gases. This noble gas is although available in various forms yet it has a limited supply to meet the demand. This is what makes the Xenon one of the most expensive noble gas.
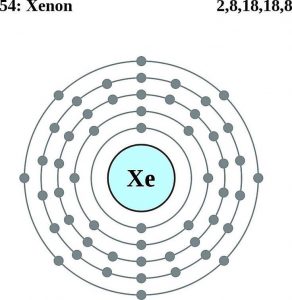
The understanding of Xenon electron configuration is essential in order to understand this noble gas element in a thorough manner. For the same purpose, we basically distribute the electrons of Xenon to its orbitals for the distribution of electrons. This distribution in results takes the shape of an equation that becomes the electron configuration of the Xenon.
How many valence electrons does Xenon have?
The electron configuration of Xenon with the same principle is [Kr] 4d¹⁰ 5s² 5p⁶ for the reference of our scholars. The electron configuration of the Xenon reveals the other major characteristics or properties of the element. For instance, with the help of electron configuration, one can easily get to understand the respective reaction of elements with the other elements.
It subsequently helps in finding the other legitimate usages of the Xenon in chemistry. There are several other properties of Xenon that you can explore in the periodic table of the element.
Electron Configuration for Xe
Well, Xenon is a highly precious noble gas in the domain of the lighting industry around the world and remains in significantly high demand. The primary usage of Xenon lies in the production of light-emitting devices such as flashlights, vehicle headlights, etc. The Xenon gas light is highly powerful and is useful even in space ships or rockets in space travel.
Furthermore, Xenon is also useful in the medical or pharmaceutical industry. It’s primarily used in the production of unique kinds of anesthesia. Xenon anesthesia is generally much more expensive than regular anesthesia. It’s useful in some special medical conditions that require the decent support of anesthesia.