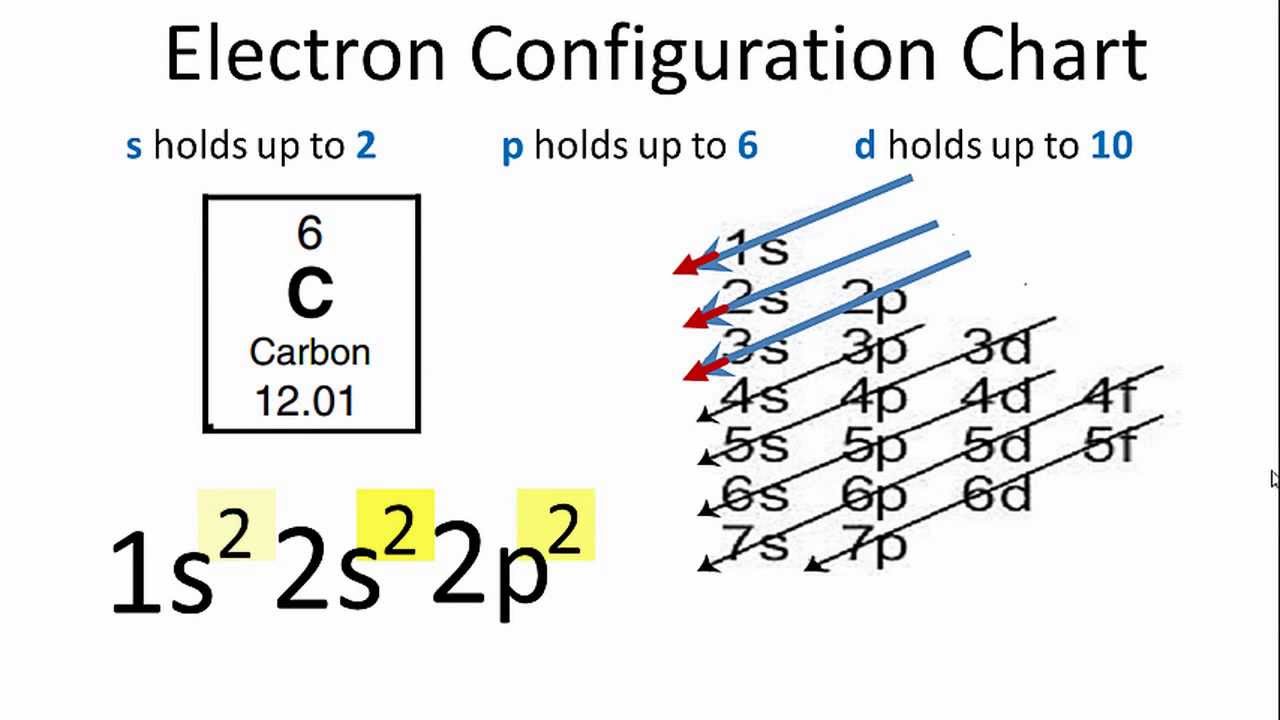
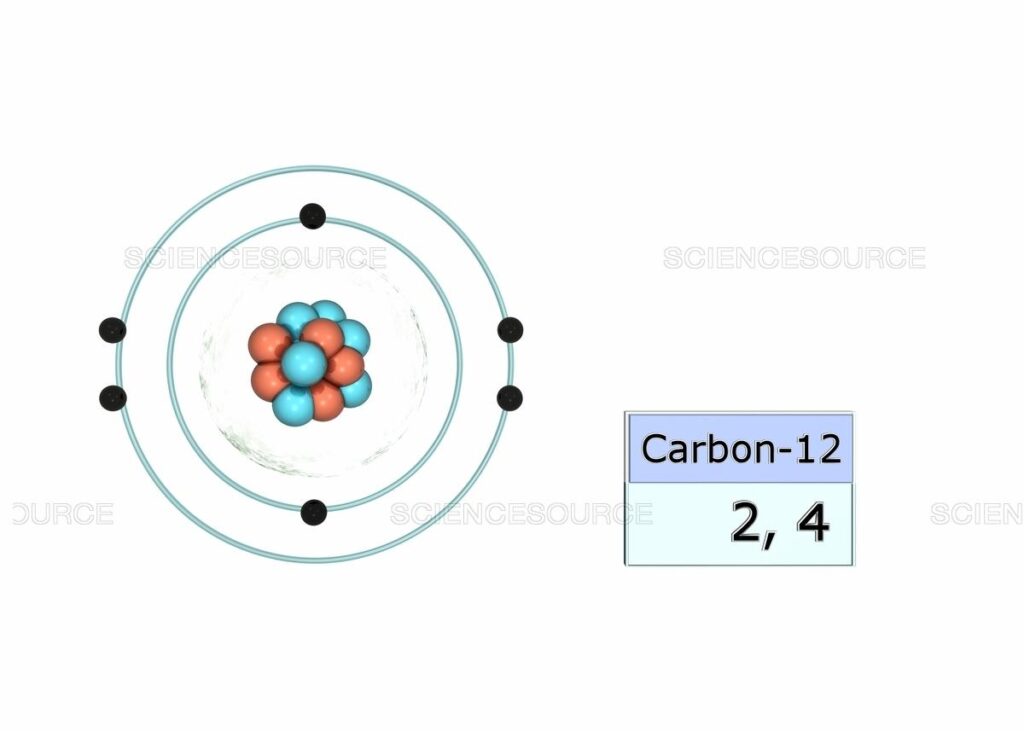
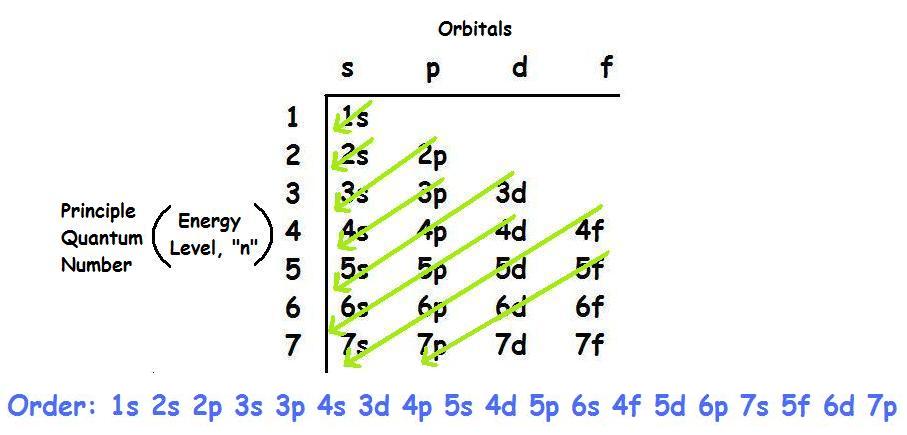
Carbon is considered to be the sixth element that has sixth electrons in the totality. In the Carbon electronic configuration of the C, the first two will be held by the 1s orbital, the next two in the 2s orbital, and the remaining ones will be held by the 2p orbital.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
Carbon Electronic Configuration
The Electronic Configuration of C given here in the given picture. You can easily view this image and also download the Electronic Configuration of C. You can check the periodic table for the position of Carbon. Carbon has 6 electrons in its orbits.
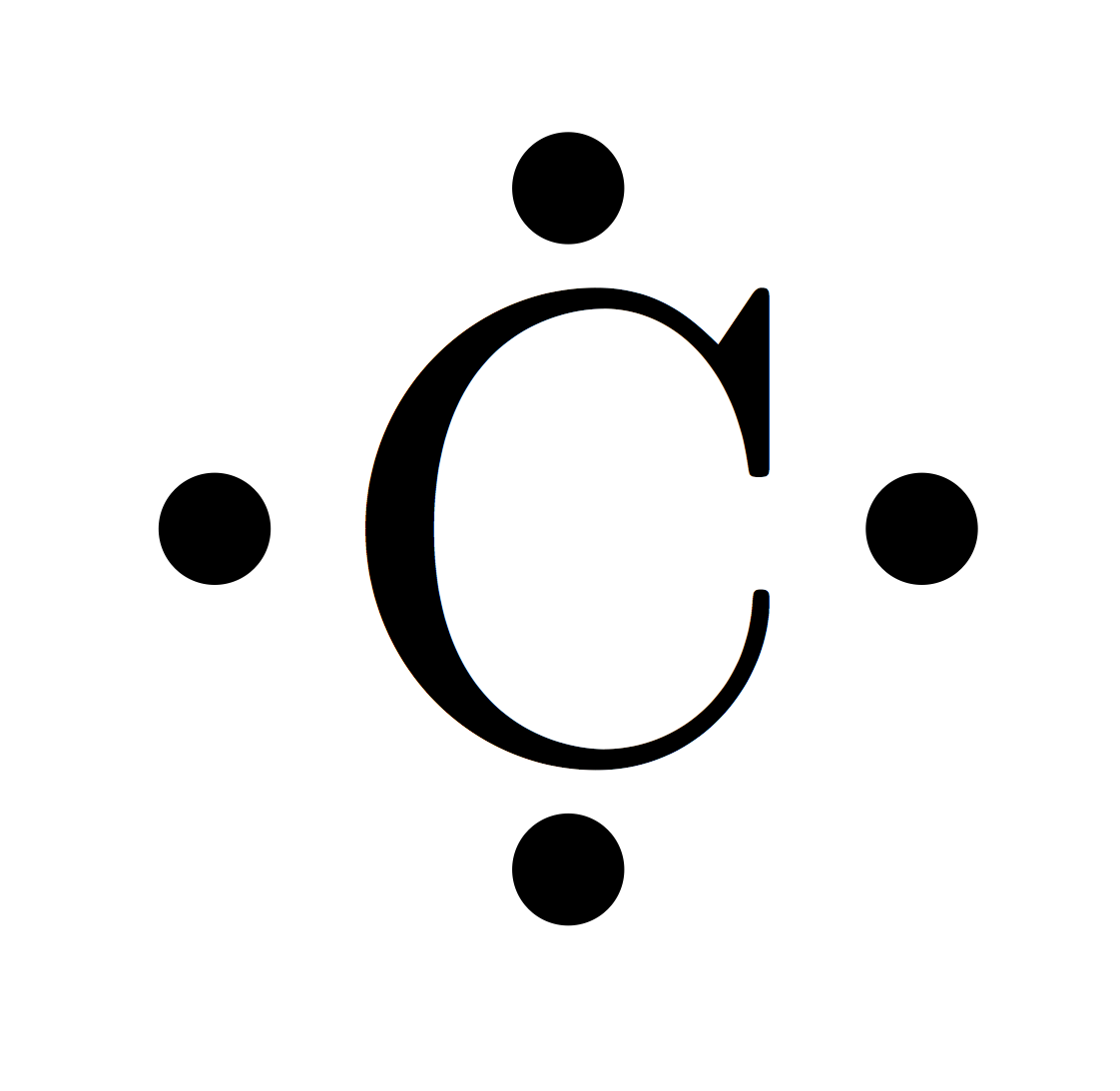
Carbon Electron Dot Diagram
The electron diagram is also known as the Lewis dot diagram since it is first used by Gilbert N. Lewis.
This diagram is used as a notation to depict the numbers of electron valence in an atom. If you are seeking the carbon electron dot diagram then here you can find the one.
Carbon Electronic Configuration
As we have elaborated above that there are sixth electrons in the C, and in the electronic configuration of the C, all these electrons are taken by the orbital. This configuration can be shown as 1s22s22p2.