Palladium Electron Configuration: Electron configuration is conducted having a chemical element in which the distribution of the electrons for that particular chemical item is made for the orbital or the molecular. It helps in understanding the chemical reaction of any chemical element.
- Bromine Electron Configuration
- Sodium Electron Configuration
- Magnesium Electron Configuration
- Aluminum Electron Configuration
- Silicon Electron Configuration
- Sulfur Electron Configuration
- Chlorine Electron Configuration
- Phosphorus Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
Today we are going to discuss the electron configuration of Palladium chemical element in this article.
Palladium Electron Configuration
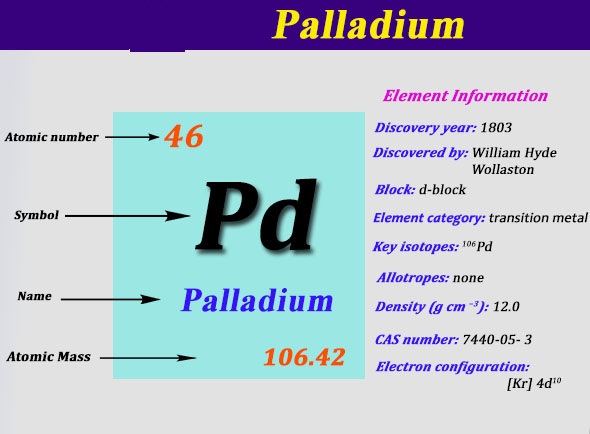
We know that palladium is a chemical element that is symbolized by the Pd and is having 46 as its atomic number. This metal was basically discovered by William Hyde in 1803. Presently this metal is famous as one of the rarest silver metals in the world.
What is The Electron Configuration of Pd?
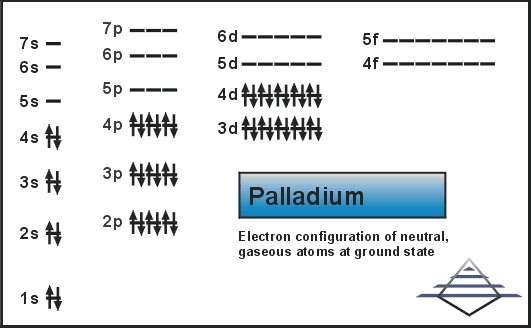
The Palladium element has the 46 electrons you can refer to the periodic table to calculate that and we further know about the S orbital, which can only retain the maximum 2 number of electrons. Further P can hold 6, d can hold 10 and the f can at last hold 14. With this equation the electron configuration becomes as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. .
Further filling the 4th shell in order to make the atom at the optimum point of stability this equation becomes [Kr] 4d10.
How Many Valence Electrons Does Palladium Have?
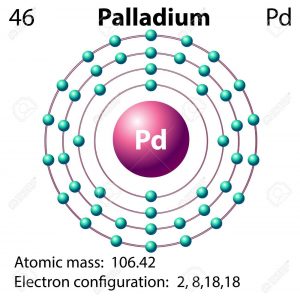
The Palladium basically has the 10 valences of the electrons which it holds in its outermost shell also known as the 4d shell. Here in this theory of having the 10 valence of electrons the palladium follows the octet rule. This rule is followed by any chemical element which holds 8 or more electrons.
Palladium Number of Valence Electrons
The palladium basically holds the 18 Electrons in the outermost shell and it is because of the metal electrons interaction which is considered to be very complex. The other reason for having the 18 Electrons by the palladium is due to the pseudo inert gas configuration nature of the palladium.
Leave a Reply