Learn and explore the Flerovium electron configuration here in our article for your learning of the chemical element. The article offers a discussion on the electron configuration and the other chemical properties of the element.
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
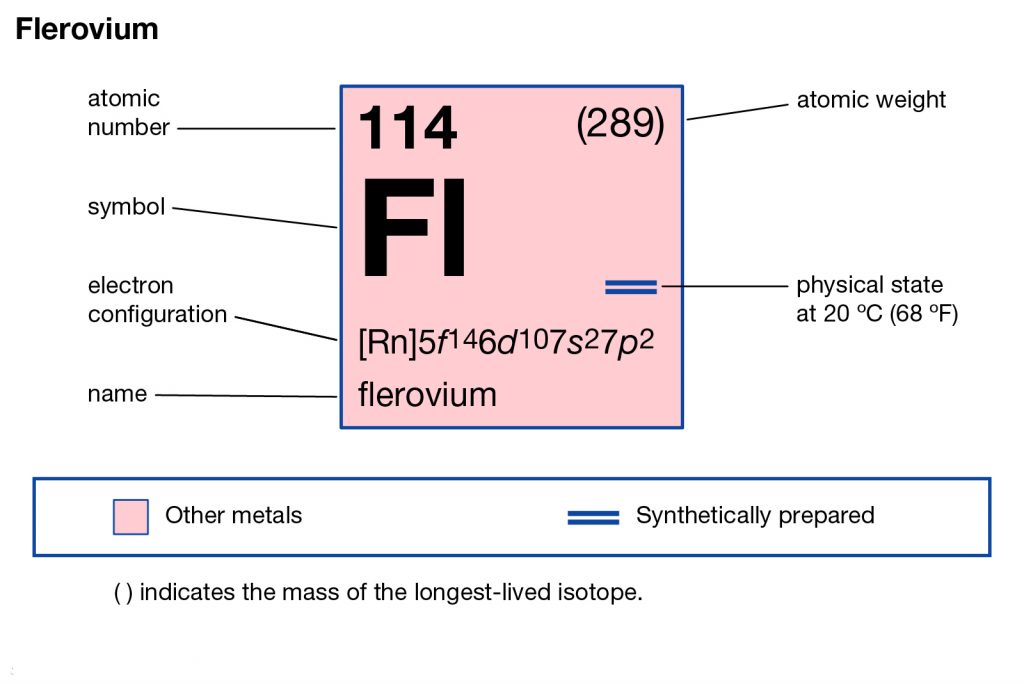
In Chemistry or science, the Flerovium is the chemical element that comes with its atomic number 114 and the symbol sign of FI. It’s basically the pure synthetic chemical element that has no free form of occurrence in nature. It means Flerovium can only be produced in the science labs for its assigned usage.
The first-ever Flerovium was made in the Russian lab by the lab called Flerov laboratory with the contributions of the Russian scientists. So, for the same reason, the name of the chemical element belongs to the same laboratory. Flerovium is a highly radioactive chemical element that is very lethal to human exposure.
Flerovium Electron Configuration
Furthermore, Flerovium is also one of the heaviest synthetic chemical elements that have very lethal radiation. This makes it much tougher to store the chemical element in a safe environment. The element belongs to the p block members in the periodic table. This block is also popular as the carbon block in chemistry and deals primarily with the heavy synthetic chemical element.
Flerovium is very volatile in its nature as it can easily react with other elements such as gold etc. The isotopes of this chemical element have a very short span of life which is hardly 2 seconds. The other chemical properties of Flerovium are still unknown in the domain of science.
Fl Electron Configuration
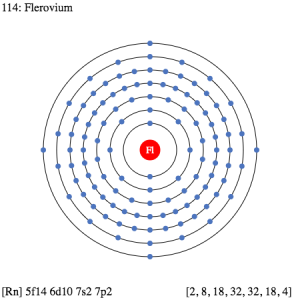
As we know that each and every chemical element in chemistry has its own electron configuration. In a similar manner, the Flerovium also has its own electron configuration that we get in the form of electron distribution. The Flerovium electron configuration is 5f146d107s27p2 in its abbreviated form which is recognized in the periodic table of elements.
The electron configuration of Flerovium is what we get when we distribute the electrons of this element to its own orbitals. This is the natural tendency of every chemical element to distribute its electrons. It helps in the overall breakdown of the element for ease of understanding purposes.
How many valence electrons does Flerovium have?
The scientists or the chemist can use the electron configuration equation to determine the other chemical properties of the element. For instance, they can figure out the chemical reaction of Flerovium with the other elements by studying its electron configuration. They can also figure out the more legit usage of the element with the electron configuration.
Well, Flevorium is still in its research phase and it has no biological role to play as of now. In fact, scientists still don’t even have the whole properties of the element as of now. All they are aware of is the highly lethal radioactive properties that make it extremely hard to harness.
Flerovium is a very expensive synthetic chemical element that has a very high production cost. For the same reason, the world has very limited availability of the element. The element also has no such usage in the commercial domain as well. We hope that the electron configuration of Flerovium would make the element a little easier for studying purposes.
Leave a Reply