Electronegativity Series: We all have studied the concept of electronegativity in our basic chemistry classes, which was primarily part of the periodic table. Reminding you of the concept once again in which we basically figure the tendency of a given chemical’s atom to attract the pair of electrons. This attraction tendency of an atom is affected by its electronegativity numbers, which is fixed for every chemical element.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
The concept of electronegativity was first given by an American chemist named Linus Pauling and today it has become an integral part of chemistry without which any chemical elements seem incomplete.
Electronegativity Series
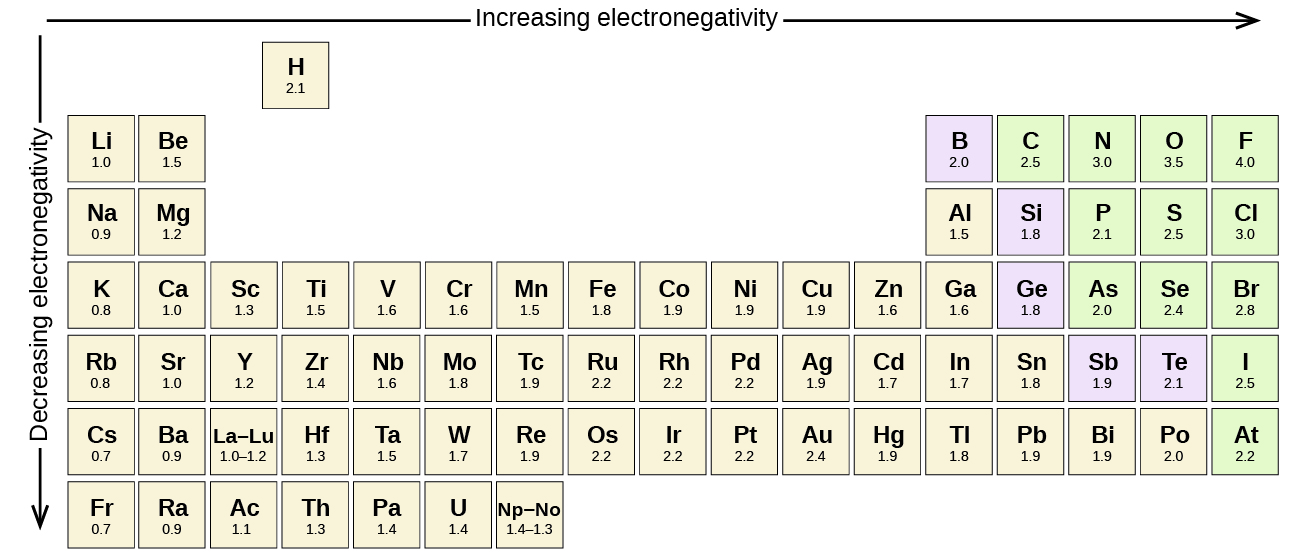
All the chemical elements have their atoms and these atoms have the tendency to attract electrons. The more is the value of electronegativity for a given chemical element the stronger would be its attraction of electrons.
Increasing Trend of Electronegativity
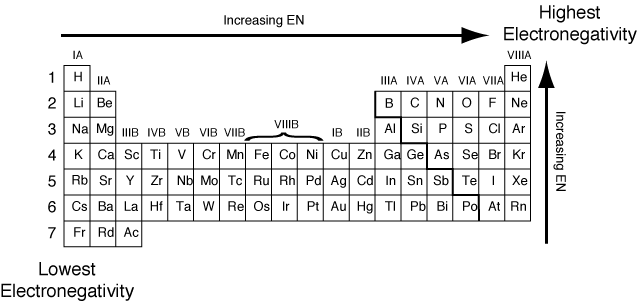
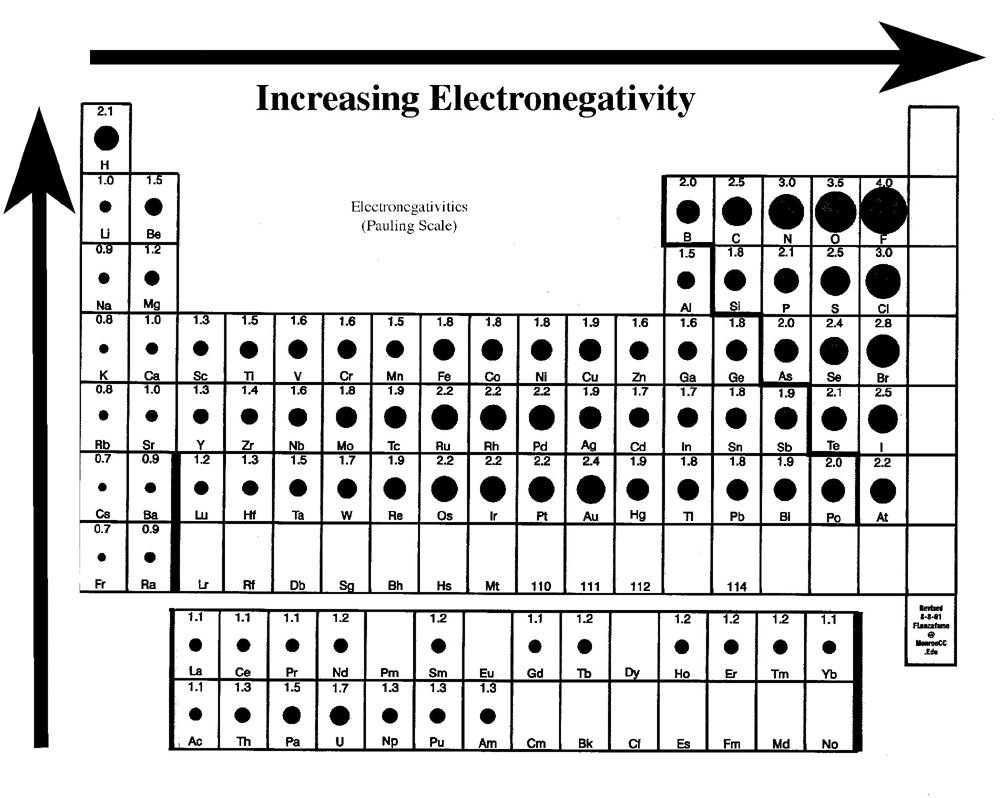
Well, if you want to check out the pattern of electronegativity for a given chemical element then you have got to take the view of the periodic table. In this table, you can find the properties of each and every chemical element which is used in Chemistry.
If you closely observe the periodic table then there you will see electronegativity properties of chemical element would be going from left to right which is actually the increasing trend of electronegativity.
In simple words in the periodic table, all chemical elements are placed in accordance to their electronegativity measures or numbers. The ones which have low electronegativity will be kept on the top left side, and the others with higher electronegativity would be kept towards the right side following the left one.
This order can be represented as F>O>N>C in the increasing trend which is used to explain the properties of organic compounds in a systematic manner. In this trend, the elements, which have lower electronegativity are known as the electropositive elements.
Leave a Reply