Boron Electron Configuration: B is a chemical element that has a chemical symbol B. The atomic number of Boron is 5. It is produced entirely by supernovae and cosmic ray spallation and not by stellar nucleosynthesis. Boron is a low-abundance element in the Earth’s crust.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
Boron Electron Configuration
It is concentrated on Earth by its property of water-solubility of the naturally occurring compounds, such as borate minerals. They are mined by the industrial process as evaporites, like as kernite and borax. The largest known deposits of boron are in Turkey, as it is the largest producer of boron minerals.
Elemental boron is a metalloid that is found in small quantities in meteoroids. However, chemically uncombined boron is not found on Earth naturally. Pure boron is produced with difficulty by the industries because of refractory contamination by other elements like carbon.
Various allotropes of boron exist; amorphous boron is a powder that is brown in color; crystalline boron is black and silver. The basic use of elemental boron is as boron filaments. Today we will tell you about the electron configuration of the boron.
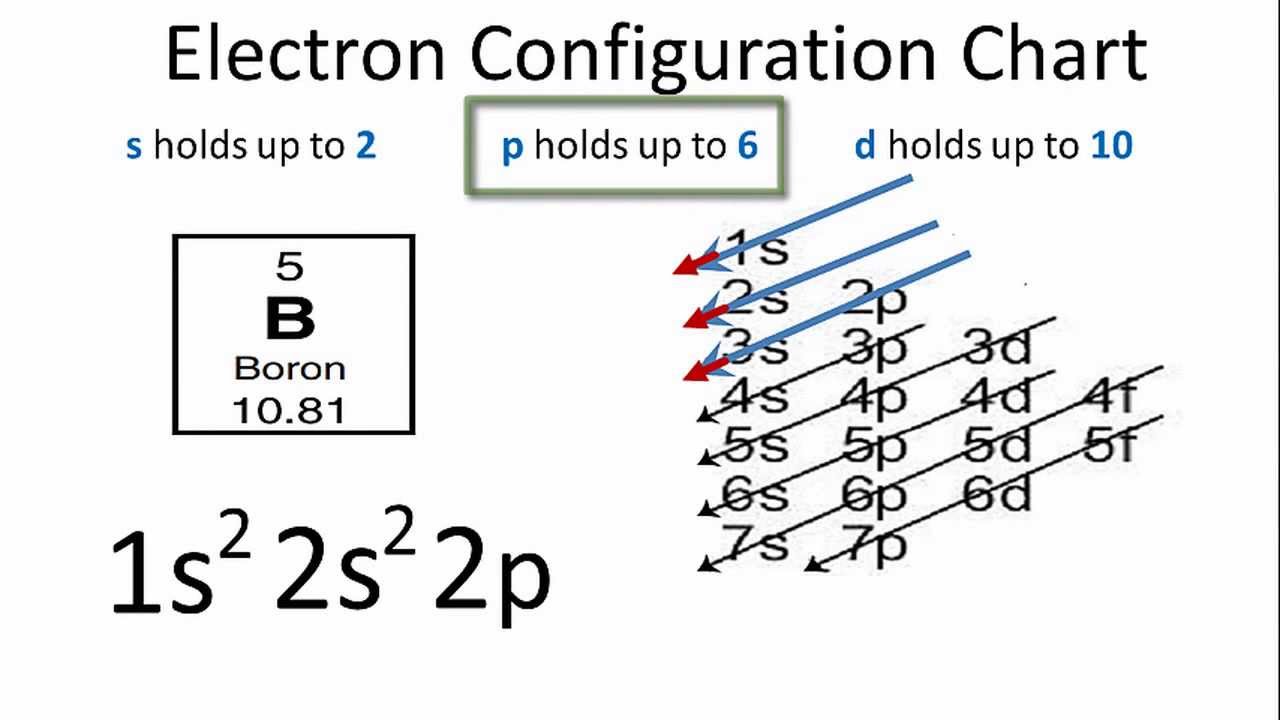
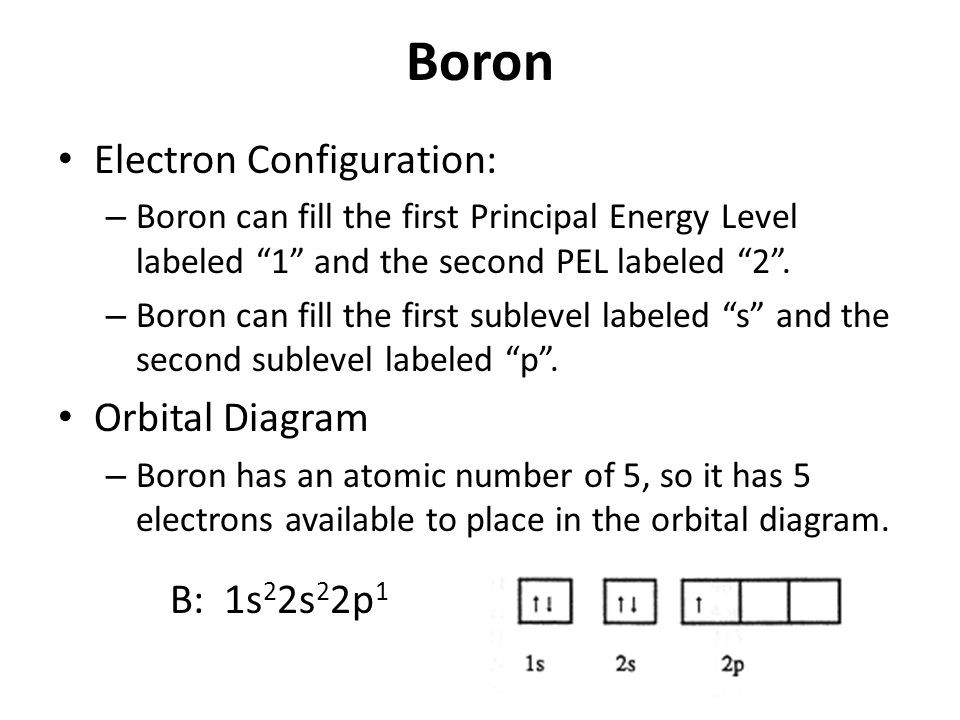
What is the Electron Configuration of Boron
[He] 2s2 2p1 is the electron configuration of B. Also the periodic table for the position of Boron.
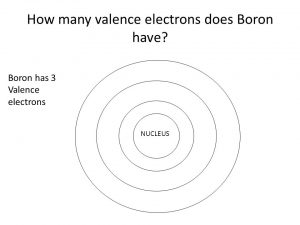
How Many Valence Electrons Does Boron Have
Boron has three valence electrons in its orbits.
Boron Number of Valence Electrons
There are three valence electrons in the outer shell of the boron. And the symbol of Boron of B.
Leave a Reply