Arsenic Electron Configuration: Chemical element, Arsenic has atomic number 33 and has the symbol “As”. Arsenic chemical occurs in many minerals like elements with a combination of Sulphur and metals. This is the pure elemental crystal. Arsenic comes in a metalloid category. This arsenic element has different allotropes which are grey in form and in metallic appearance.
- Hydrogen Electron Configuration
- Helium Electron Configuration
- Lithium Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
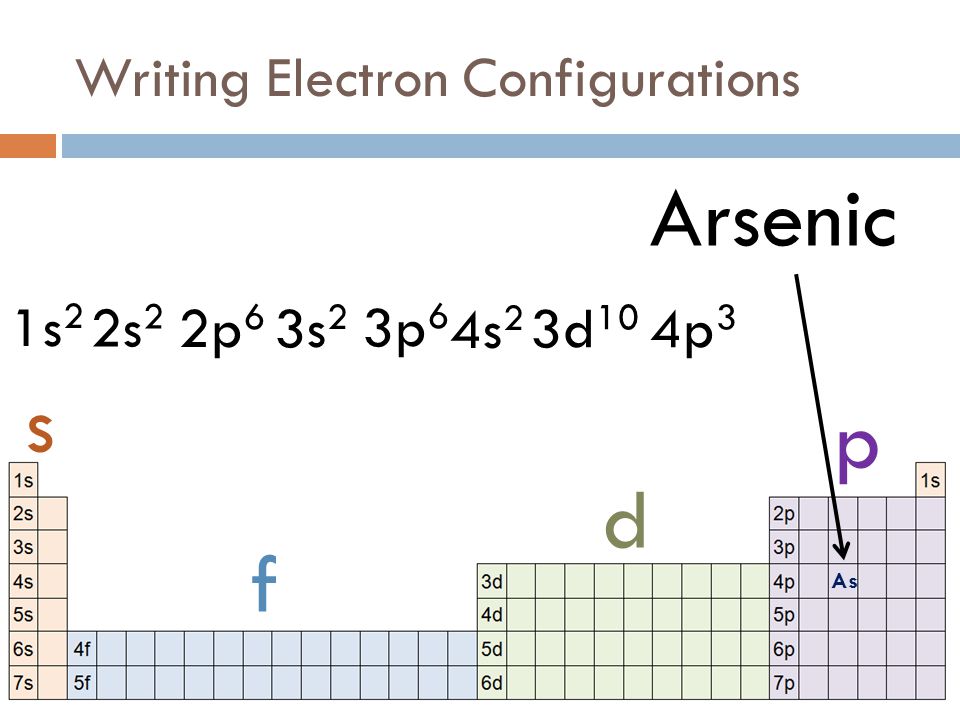
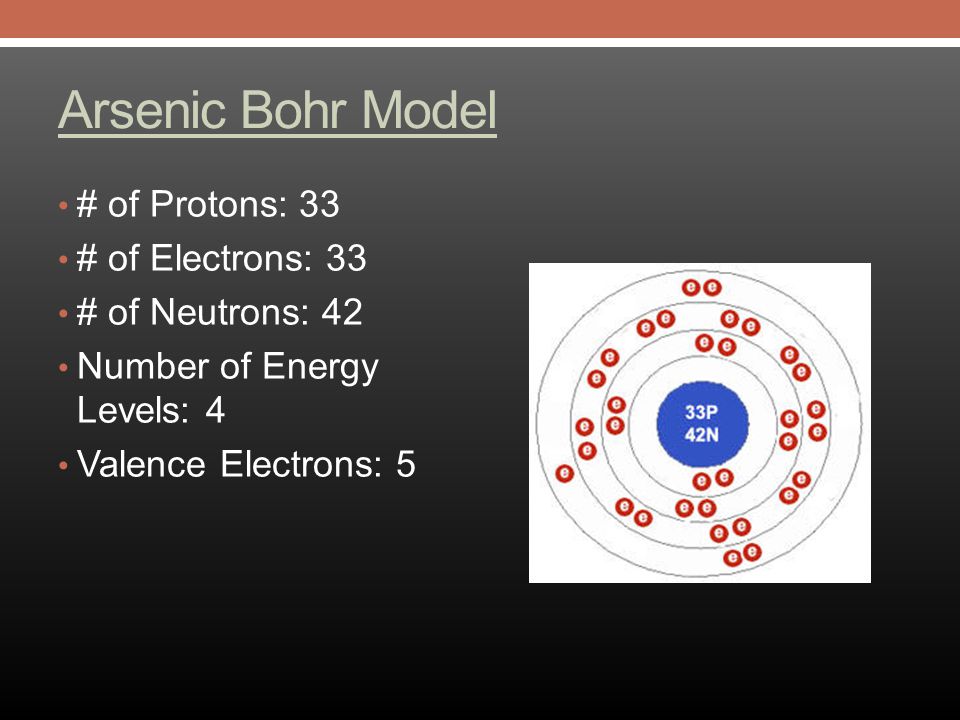
Arsenic Electron Configuration
An electronic configuration is a number of electrons in the orbits of molecules and atoms. Well, in the case of Arsenic there are 33 electrons which lie in 4 shells. The distribution of electrons in shells is: 2, 8, 18, 5, and this electronic configuration can be written as:
1s22s22p63s23p64s23d104p3 or [Ar] 3d104s24p3
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
Arsenic Number of Valence Electrons
Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. In the case of Arsenic, there are 5 electrons in the outer or fourth shell.
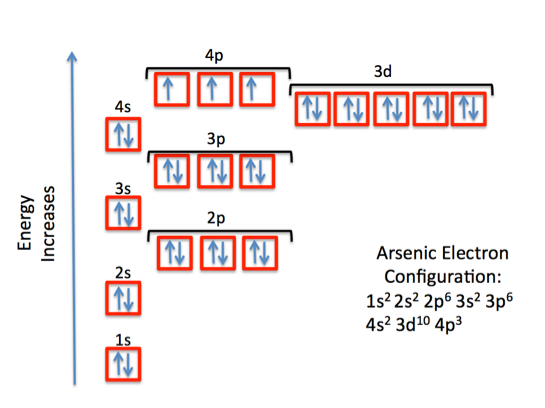
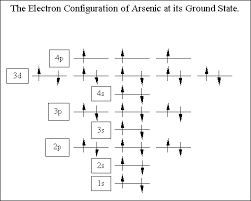
Ground State Electron Configuration For Arsenic
The ground-state electron configuration of an atom can represent in the form of pairs of electrons which can also be represented in the orbital diagram as well. And this ground-state electron configuration for Arsenic is defined as:
1s22s22p63s23p64s23d104p3
What is The Electron Configuration of Arsenic
The electronic configuration of an Atom or molecule is the number of e– (electrons) in the orbit or shells. And electrons of Arsenic is 33, and its electron configuration of arsenic is 1s22s22p63s23p64s23d104p3
How Many Valence Electrons Are in Arsenic
Valence electrons are the electrons which are present in the outer shell or orbit. And there are 5 valence electrons in Arsenic.
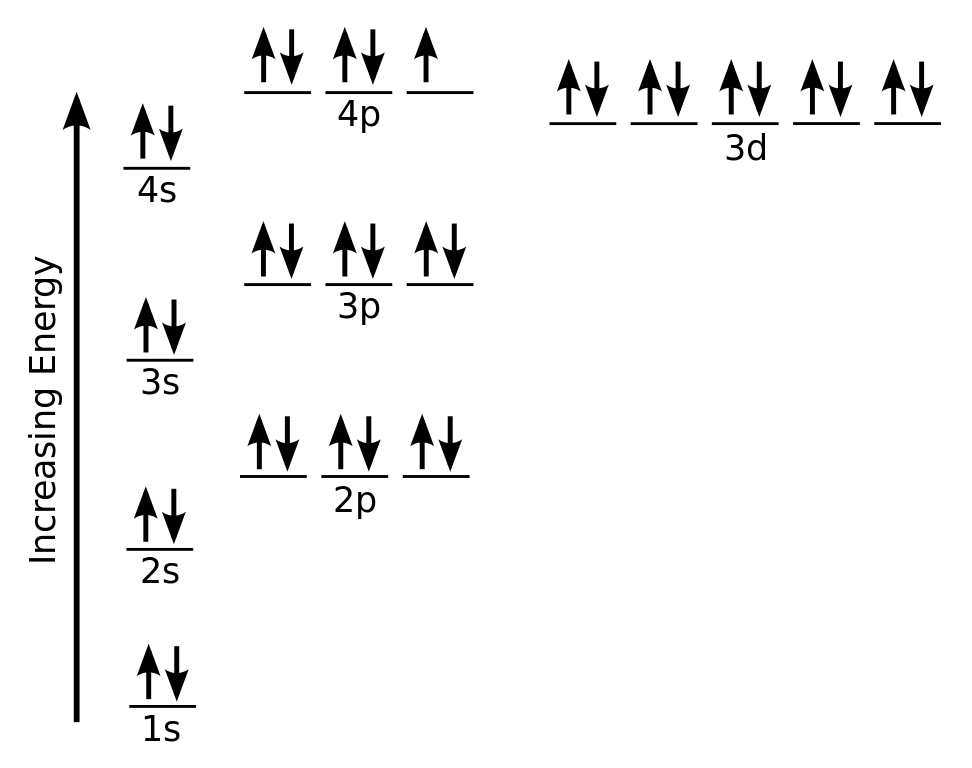
What is The Orbital Diagram For Arsenic
The orbital diagram consists of a pair of electrons in one box that is in the form of arrows (like ↑↓ and on the basis of a number of electrons pairs distribution of electrons becomes easy. On the basis of pairs and unpaired electrons, an orbital diagram is formed. In case of Arsenic there are 3 unpaired boxes of electrons of 4p3 and rest 30 electrons in the pairs.
Leave a Reply