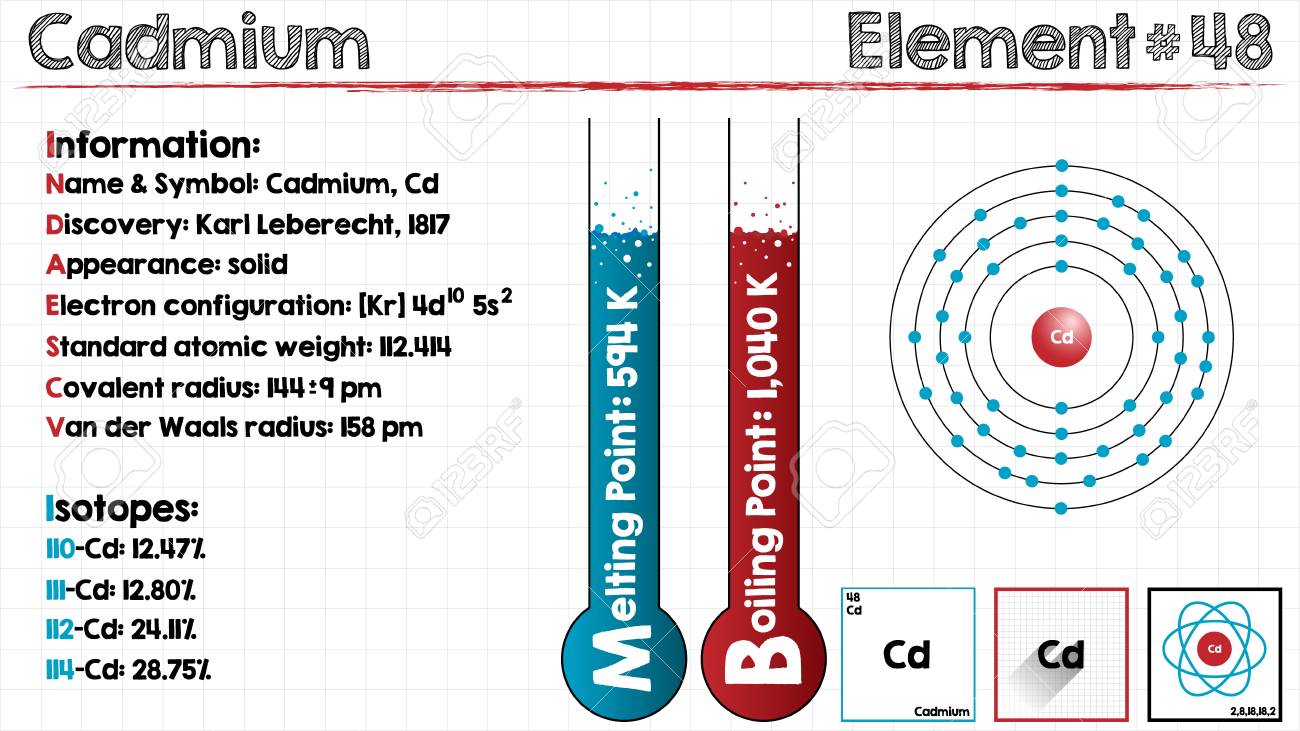
Cadmium Electron Configuration: Cadmium is a chemical element which has a chemical symbol Cd. The atomic number of the Cadmium is 48. It is soft, bluish-white metal and is chemically similar to the two other stable metallic elements in group 12 of the periodic table, namely mercury and zinc. Similar to zinc, it shows oxidation state +2 in most of its compounds and similar to mercury, it has a low melting point than the transition metals of groups 3 and group 11.
- Bromine Electron Configuration
- Sodium Electron Configuration
- Magnesium Electron Configuration
- Aluminum Electron Configuration
- Silicon Electron Configuration
- Sulfur Electron Configuration
- Chlorine Electron Configuration
- Phosphorus Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
Along with its congeners in group 12 they are often not considered transition metals, because of common oxidation states and that they do not have partly filled d or f electron shells in the elemental. On an average concentration of cadmium in Earth’s crust is ranging between 0.1 and 0.5 parts per million. It was discovered simultaneously by Stromeyer and Hermann in 1817 in Germany, as an impurity in zinc carbonate.
It occurs as a smaller component in many zinc ores and is also a byproduct of zinc production. It was used for a long time as a corrosion-resistant plating on steel. Its compounds are used as orange, red, and yellow pigments, to stabilize plastic and to colour glass, and. Its use is generally decreasing as it is toxic and is specifically listed in the European Restriction of Hazardous Substances.
Today we are here to share the information about the electron configuration of the Cadmium which will help you to understand it in a better way. If you are also here for the same then you are in the right place. Please see the full post below for more information.
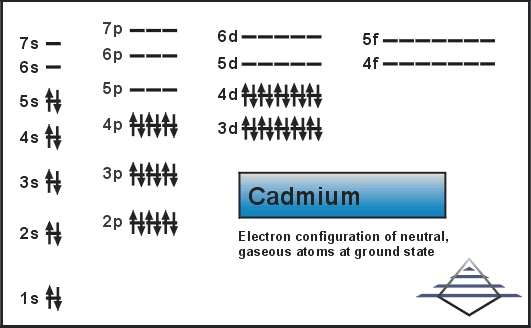
What is the Electron Configuration of Cadmium?
Kr 4d10 5s2 is the electron configuration of Cadmium.
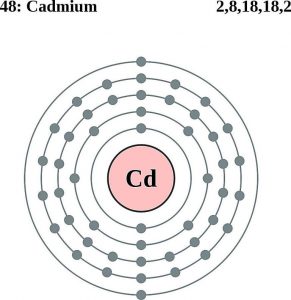
How Many Valence Electrons Does Cadmium Have
There are two valence electrons in the outer shell of the cadmium.
Cadmium Number of Valence Electrons
Cadmium has only two valence electrons in it is the outer shell.
We hope you find this article useful. Please let us know if you have any queries.
Leave a Reply