Learn about the Tennessine electron configuration here in our article and explore this chemical element in an extensive manner. The article will provide some useful information on the electron configuration and other chemical properties of Tennessine.
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
Tennessee belongs to the family of artificial or synthetic chemical elements that have significant recognition in chemistry. The element has the atomic number 117 and the symbolic sign of Ts. Tennessine is basically the second heaviest chemical element that belongs to the 7th period in the periodic table.
Tennessine Electron Configuration
This is yet another synthetic chemical element whose discovery credit goes to the Russian lab and by an experimental Russian scientist. It was discovered recently in the year of 2010 and that makes it the most recently discovered chemical element. Later there were several other experiments that took place jointly by the American and Russian scientists.
Furthermore, Tennessine is a very volatile chemical element that may have a very quick reaction with the other elements. The element may have the oxidation states such as -1,+1,+3,+5 with its stable isotope. The high radioactivity of the element makes it very lethal and toxic to its open exposure.
Electron Configuration for Ts
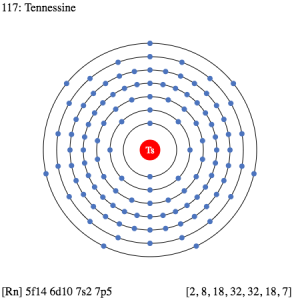
The electron configuration of Tennessine is one of its highly significant properties. The electron configuration is basically the process by which the element distributes its electrons to its own atomic orbitals. The process is extremely significant as it helps in the overall breakdown and further analysis of the element.
So, we basically have the Tennessine electron configuration as [Rn] 5f146d107s27p5 in its short abbreviated form. This electron configuration of the Tennessine helps the scientists in finding the other chemical properties of the element. They eventually aim to find the more practical usage of the element in the real world. Here is why the Tennessine electron configuration is useful in the overall study and analysis of the element.
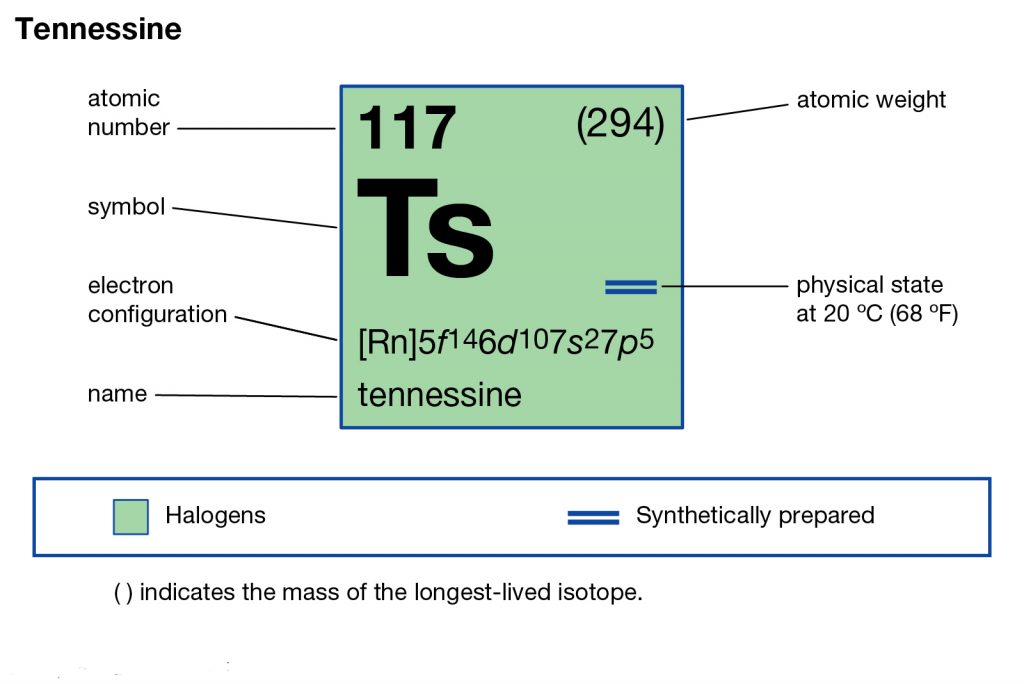
Figuring out the most precise chemical properties of the element and seeking its best pair that has the similar or same electron configuration. Electron configuration is mandatory so as to find the valency of the element. It also helps in the interpreting process for the atomic spectra of elements.
So, all these factors make the electron configuration extremely significant for the Tennessine chemical element. You can check all the other properties of the element in its periodic table.
How many valence electrons does Tennessine have?
Well, just like any other chemical element Tennessine also has its own relevance and significance in the scientific domain. The element is still in its very early research phase and therefore has no known biological usage as of now. Being a synthetic chemical element it has its own radiation which is highly toxic to human exposure.
Moreover, it has very quick decaying chemical properties that make it critical for its proper storage. The element generally incurs very high production costs so it has very limited availability. As per the current availability of research on the element, it’s supposed to bring its utility in nuclear power. So, the long-term utility prospect of the Tennessine is very bright from here.
Leave a Reply