Vanadium Electron Configuration: Vanadium is a chemical element. It has a symbol V. The atomic number of Vanadium is 23. It is a silvery-grey, hard, malleable, and ductile transition metal. This metal is rarely found in nature, but if it is isolated once artificially.
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Carbon Electron Configuration
- Oxygen Electron Configuration
- Manganese Valence Electrons
- Helium Electron Configuration
- Beryllium Electron Configuration
Vanadium Electron Configuration
However, the formation of an oxide layer (passivation) stabilizes the free metal to some extent against further oxidation. The Vanadium was discovered by Andrés Manuel del Río in 1801 in Mexico. He discovered it by analyzing a new lead-bearing mineral he named “brown lead”, and also presumed that its qualities are due to the presence of a new element, which he called erythronium.
- Chlorine Valency
- Argon Valency
- Potassium Valency
- Calcium Valency
- Technetium Valency
- Titanium Valency
- Vanadium Valency
- Chromium Valency
- Manganese Valency
- Cobalt Valency
- Copper Valency
- Gallium Valency
- Arsenic Valency
Electron Configuration For Vanadium Ion
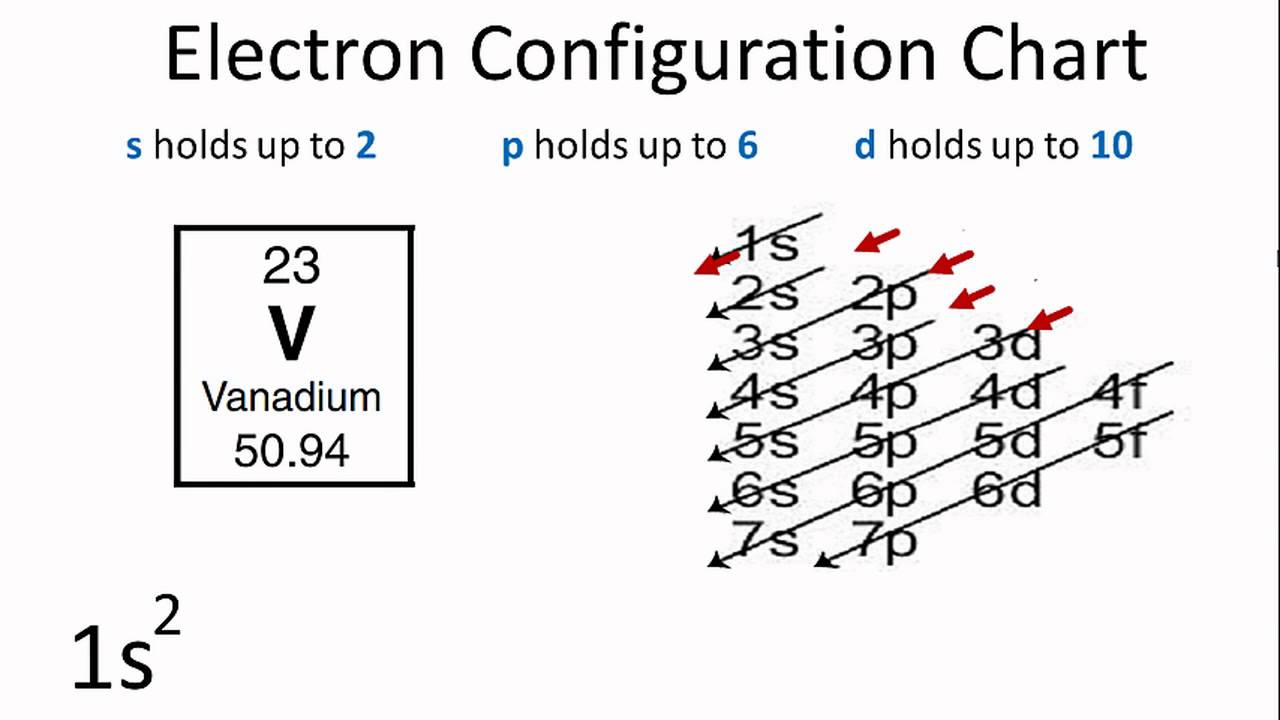
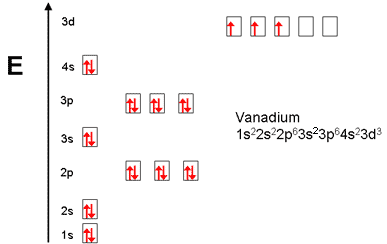
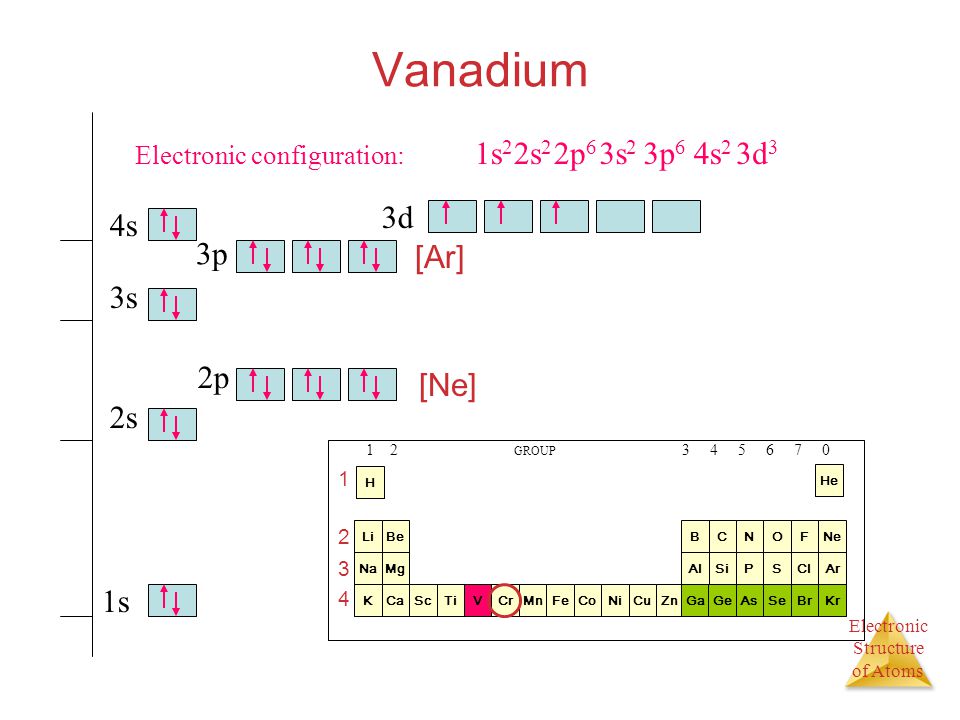
The electron configuration for V can be written as Ar 3d3 4s2. For better understanding see the pictures below;
Vanadium Number of Valence Electrons
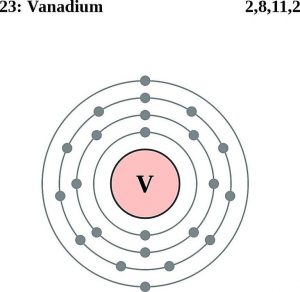
The vanadium number of valence electrons is 5 and it belongs to the 5th group.
Ground State Electron Configuration of V
Ground state electron configuration depends upon the pair distribution of electrons. And for Vanadium ground state electron configuration is written as [Ar]3d34s2
What is The Electron Configuration of V
See the picture below for the electron configuration of V.
How Many Valence Electrons are in Vanadium
Vanadium has 4 valence electrons. You can see the first picture of this.

Leave a Reply