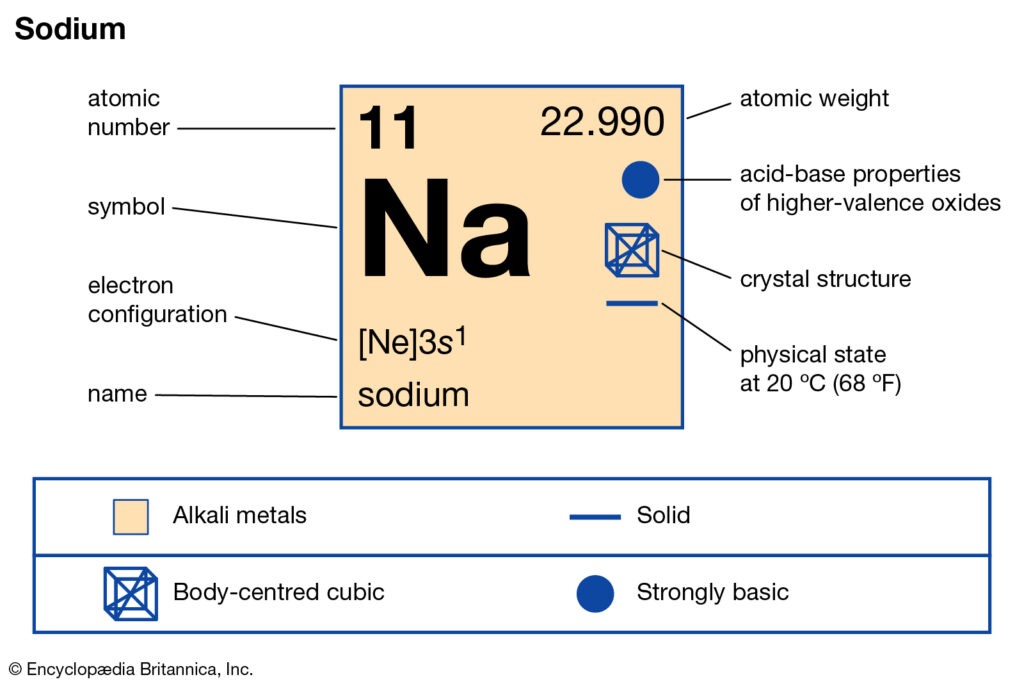
Sodium Valence Electrons: Sodium is a chemical element that has the symbol ‘Na’ which comes from the Latin word Natrium and has an atomic number 11. Sodium is a highly reactive metal that is soft in nature and silver-white in color. Sodium chemical element comes in group 1 of periodic table. It is the 6th element in the earth’s crust.
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Nobelium Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Gold Valence electrons
- Mercury Valence electrons
- Lead Valence electrons
- Bismuth Valence electrons
- Radon Valence electrons
- Radium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium valence electrons
Sodium exists in so many minerals like feldspars, sodalite, and rock salt. There are many salts of sodium which is soluble in water. Sodium Chloride is the most used compound of sodium i.e., common salt. It is metal which we can easily cut with the help of knife and also, it is a good conductor of electricity and heat because of its 1 valency in the valency shell.
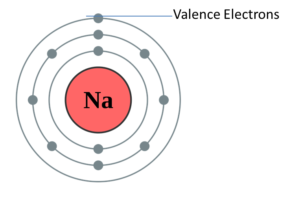
Sodium Valence Electrons Dot Diagram
Lewis’s electron dot diagram is also known as the valence electrons dot diagram. The diagram shows the valence electrons of the atom which is located as a dot as a circle form around the element. What is the number of dots known here? The number of dots in the diagram is known as the number of valence electrons in the atom. These dots are located around the symbol of the element in the right, left, above and below of the symbol. Here, is the dot diagram of sodium valence electrons for you. From which you can easily clear out your concept of the particular chemical elements. So, you can check out any of the chemical element’s diagram from here itself.
Valency of Sodium – Na
As we know that the sodium has 11 electrons. It is arranged in this way that 2 electrons are located in a first shell, 8 electrons are located in a second shell, and 1 electron in the third shell. And with the help of the last number, we can recognize the number of valence electrons of the sodium. So, the amount of valency of the sodium is 1.