Fluorine Electron Configuration: Electron configuration is an important theorem of science and if we talk about this electron configuration in quantum chemistry or atomic physics then we would find a simple understanding of it.
- H Electron Configuration
- He Electron Configuration
- Li Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- C Electron Configuration
- Neon Electron Configuration
- Vanadium Electron Configuration
- Cl Electron Configuration
As per which electron configuration explains the distribution of electrons for the atom or the molecule in the context of molecular orbital and this electron configuration can be made for the atom of any chemical element.
Today in this article we are going to talk about the electron configuration for the Fluorine.
Fluorine Number of Valence Electrons
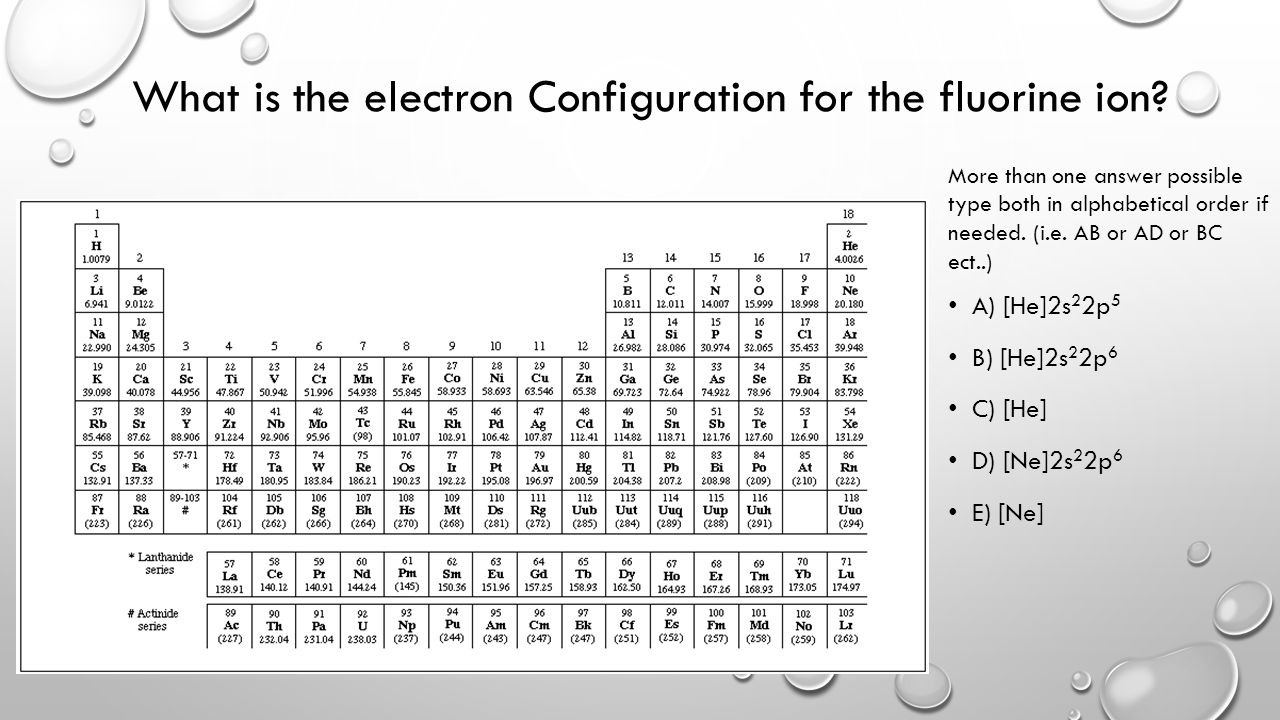
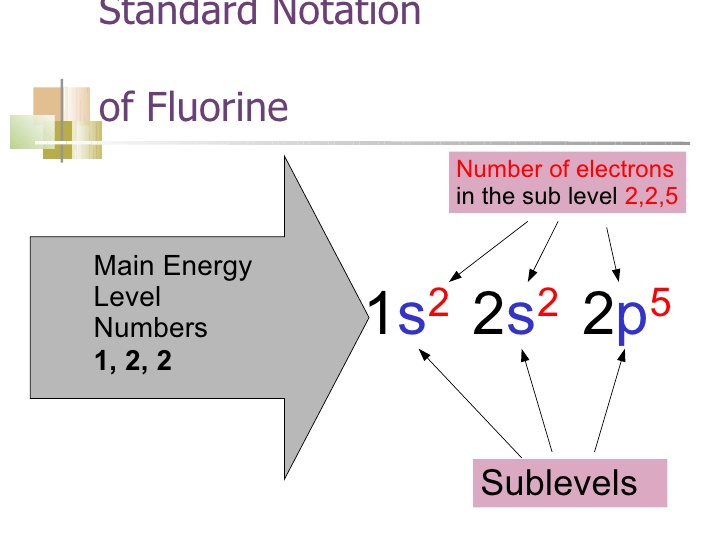
The fluorine atom basically holds the 7 valence electrons and it also belongs to the group 7 element. Further, the fluorine has the 9 atomic number and the electronic configuration of the Fluorine can be written as He] 2s2 2p5.
Since the Fluorine holds the 7 electrons and this is why it needs to complete its octet within one electron in order to fill out the outer orbits. Hence Fluorine in resultant gains one electron which makes its Valency to be as -1.
What is Electron For Fluorine
As we have explained above the whole equation of Fluorine continuing it further the Fluorine is basically the 9th element which is having the 9 electrons in totality. In this whole equation, the first two electrons of the Fluorine will go to the 1s orbital and the next 2 electrons will be held by the 2s orbital. The remaining 5 will go to the 2p orbital.
Electronic Configuration For Fluorine ion
The electronic configuration for the Fluorine ion is 1s22s22p5 and in this configuration, Fluorine needs 1 electron so as to complete the 2p orbital. This 1 electron by the Fluorine will be acquired in this whole process of fluoride ion.