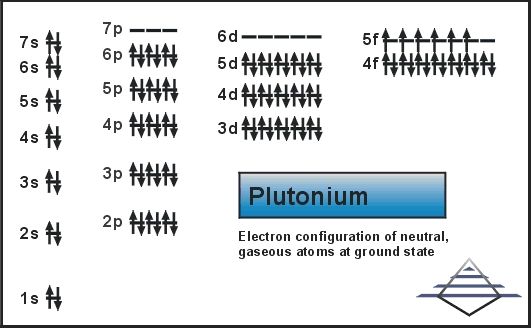
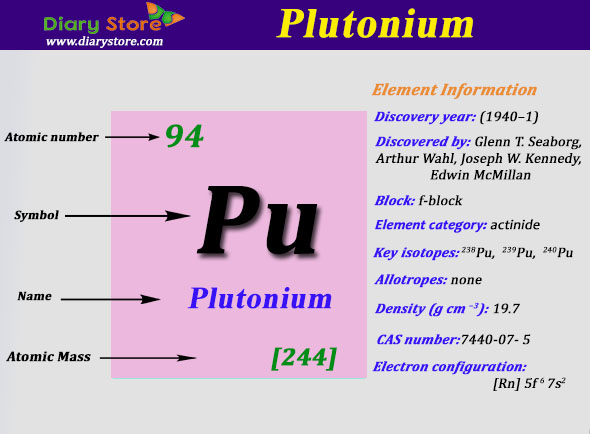
Plutonium Electron Configuration: Plutonium is a radioactive chemical element that has a chemical symbol, Pu. The atomic number of Plutonium is 94. It is an actinide metal that has a silvery-grey appearance which tarnishes when it is exposed to air, and when oxidized forms a dull coating.
- Hydrogen Electron Configuration
- Helium Electron Configuration
- Lithium Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
The element normally shows four oxidation states and six allotropes. It reacts with halogens, carbon, silicon, nitrogen and hydrogen. When it is exposed to moist air, it forms hydrides and oxides which can expand the sample up to 70% in volume, which in turn flake off as a powder which is pyrophoric.
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
Plutonium Electron Configuration
Plutonium is radioactive which can accumulate in bones, and makes the handling of plutonium dangerous. It was named after the planet, Pluto. It is the element that has the highest atomic number to occur in nature.
Plutonium trace quantities arise in natural uranium-238 deposits when U-238 captures neutrons. It is much more common on Earth as a product of beta decay and neutron capture. Today we are going to share you the information of the Plutonium.
What is the Electron Configuration of Plutonium
Rn 5f6 7s2 is the electron configuration of the Plutonium.
How Many Valence Electrons Does Plutonium Have
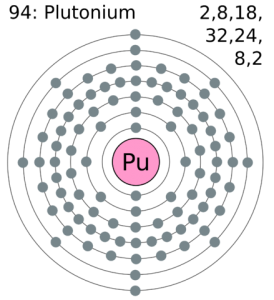
There are two valence electrons in the outer shell of the Plutonium.
Plutonium Number of Valence Electrons
Plutonium has two valence electrons in its outer shell. You can see this in a given image.