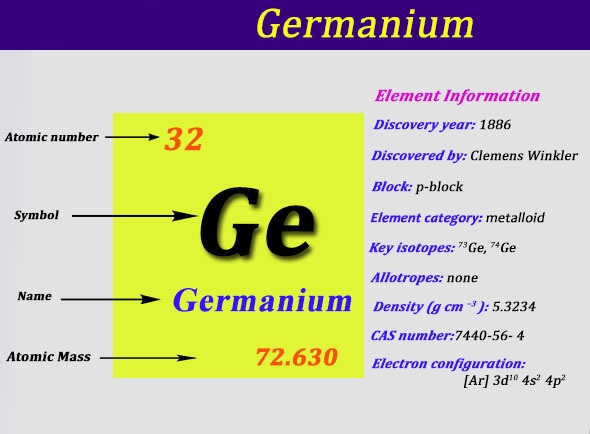
Germanium Electron Configuration: Ge (Germanium) is a chemical element that has a chemical symbol Ge. The atomic number of Germanium is 32. It is a hard, lustrous, greyish-white metalloid of the carbon group. It is similar chemically to its group neighbors silicon and tin.
- Hydrogen Electron Configuration
- Helium Electron Configuration
- Lithium Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
Germanium Electron Configuration
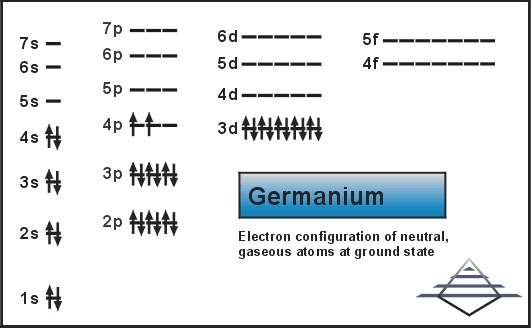
Its pure form is a semiconductor with its appearance similar to elemental silicon. Same as silicon, it naturally reacts and forms complexes within nature oxygen. Today we will provide you with the electron configuration of the Ge.
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
What is the Electron Configuration of Ge
Ar 3d10 4s2 4p2 is the electron configuration of Ge.
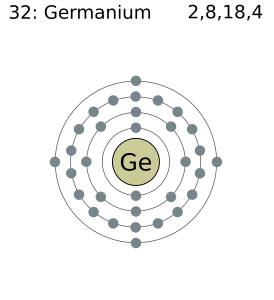
How Many Valence Electrons Does Germanium Have
Germanium has four valence electrons in its outer shell. As you can see in the given picture. Many more electron Configuration of many elements also provided here.
Germanium Number of Valence Electrons
There are four valence electrons in the outer shell of the Germanium.
Ground State Electron Configuration of Ge
[Ar].3d10.4s2.4p2 is the ground state electron configuration of Ge.
Leave a Reply