Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high strength. It is resistant to corrosion in aqua regia, sea water, and chlorine.
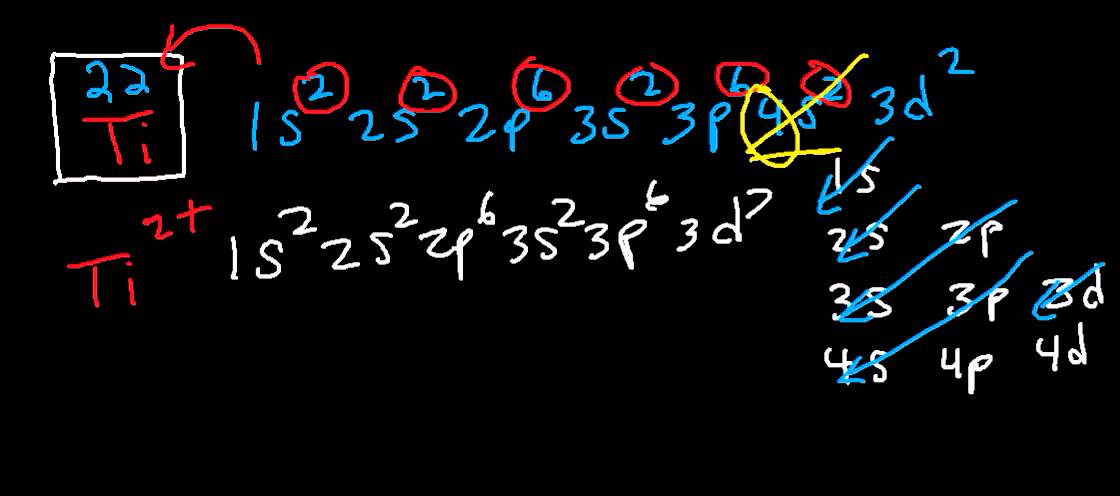
Titanium was discovered by William Gregor in Cornwall, Great Britain in 1791. It was named by Martin Heinrich Klaproth after the Greek mythology’s Titans. The element exists in a number of mineral deposits that are principally ilmenite and rutile, ilmenite and are widely distributed in the lithosphere and Earth’s crust.
Titanium Electron Configuration
It is found in all living things, rocks, water bodies, and soils. Titanium metal is extracted from the principal mineral ores by the Hunter processes and Kroll. Its most common compound which is titanium dioxide is a famous photocatalyst that is used in the manufacture of white pigments.
- Potassium Valency
- Calcium Valency
- Technetium Valency
- Titanium Valency
- Vanadium Valency
- Chromium Valency
- Manganese Valency
- iron Valency
- Cobalt Valency
- Nickel Valency
- Copper Valency
- Zinc Valency
- Gallium Valency
- Germanium Valency
- Arsenic Valency
- Selenium Valency
Other titanium compounds include titanium tetrachloride(TiCl4), it is a component of smoke screens and titanium trichloride (TiCl3) and catalysts, It is used as a catalyst in the production of polypropylene. Today we are going to give you full information on the electron configuration of Ti.
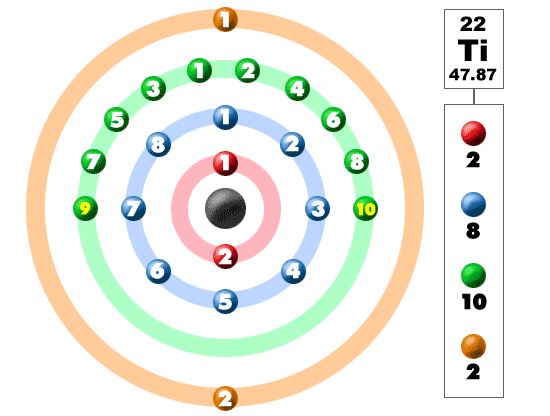
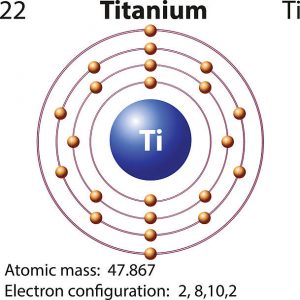
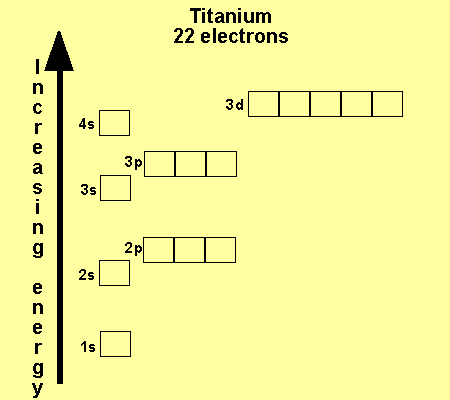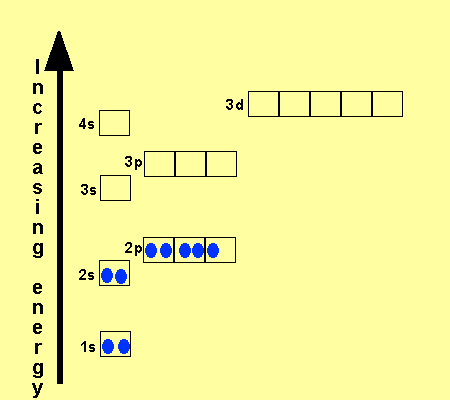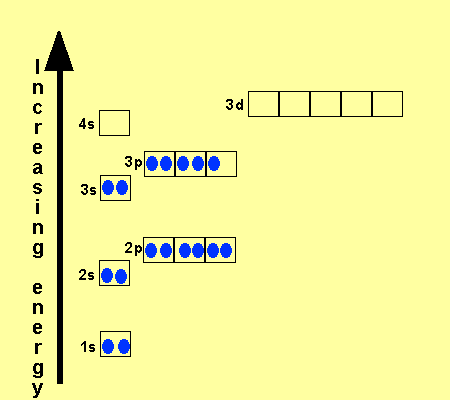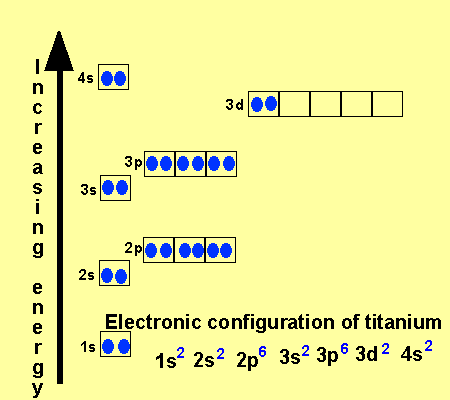
What is the Electron Configuration of Titanium
[Ar] 3d2 4s2 is the electron configuration of Titanium.
How Many Valence Electrons Does Titanium Have
There are 4 Valence electrons in the outer shell of Titanium.
Titanium Number of Valence Electrons
Titanium has 4 valence electrons in its outer shell.
Leave a Reply