Copper Electron Configuration: Copper is a chemical element that has the symbol Cu that came from a Latin word called cuprum. The atomic number of copper is 29. It is a ductile, malleable, and soft metal that has a very high electrical and thermal conductivity.
Copper Electron Configuration
The surface of pure copper that is freshly exposed has a reddish-orange colour. It is used as a conductor of electricity and heat, as a constituent of various metal alloys and as building materials, like sterling silver is used for making jewellery, cupronickel is used for making marine hardware and coins, and constantan is used in strain gauges. Today we are going to tell you about the electron configuration of the Cu.
- Cl Valence Electrons
- Ar Valence Electrons
- K Valence Electrons
- Ca Valence Electrons
- Technetium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Fe Valence Electrons
- Co Valence Electrons
- Ni Valence Electrons
- Cu Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- selenium Valence Electrons
Copper Number of Valence Electrons
There are two valence electrons in the copper.
Full Electron Configuration For Copper
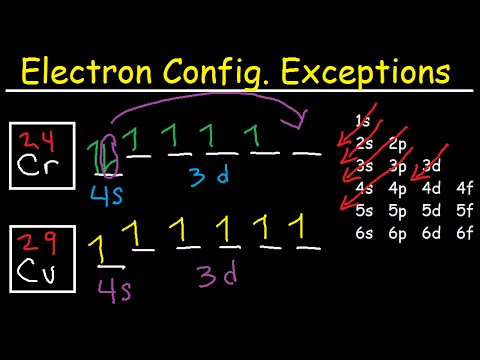
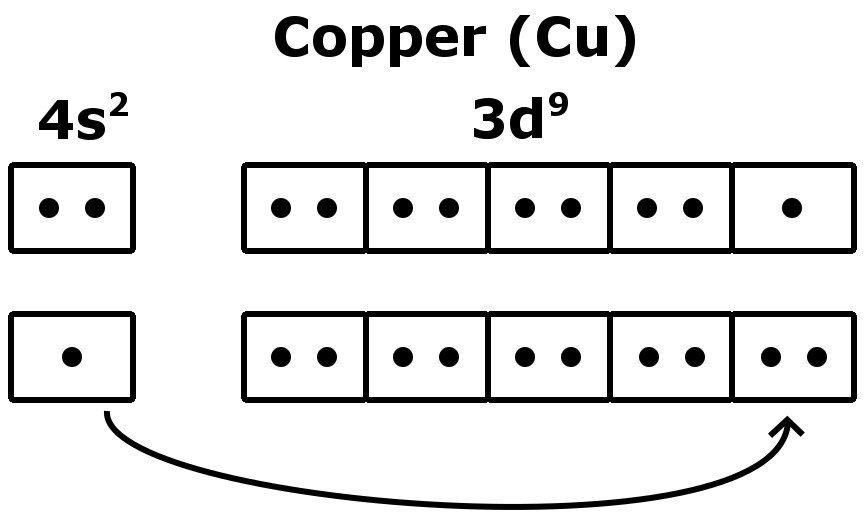
The full electron configuration for copper is [Ar] 3d10 4s1.
What is The Electron Configuration of Copper
1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of Cu.
If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The only difference is at the end of the configuration that is in the 3d and 4s shells. The atoms of copper have enough electrons which completely fill the 3d sub-shell.
How Many Valence Electrons Does Copper Have
Copper has the total of two valence electrons.


Leave a Reply