Calcium Electron Configuration: Greetings for the day everyone. Today we will talk about a chemical element, Calcium which has a symbol of Ca and its atomic number is 20. Calcium is alkaline earth metal and an also reactive metal which forms a dark-colored oxide-nitride layer when it is exposed to air. Its chemical and physical characteristics are almost similar to its heavier homologues barium and strontium. and barium.
- H Electron Configuration
- He Electron Configuration
- Electron Configuration for Li
- Beryllium Electron Configuration
- Boron Electron Configuration
- Electron Configuration for C
- Fluorine Electron Configuration
- Neon Electron Configuration
- Vanadium Electron Configuration
- Clorine Electron Configuration
Calcium Electron Configuration
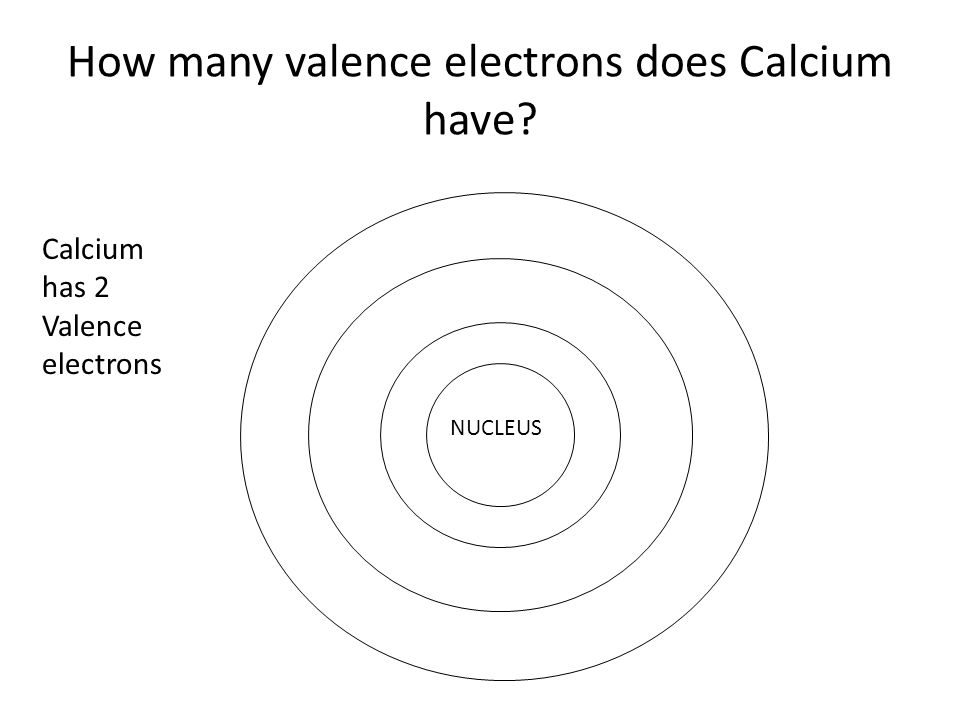
Calcium Number of Valence Electrons
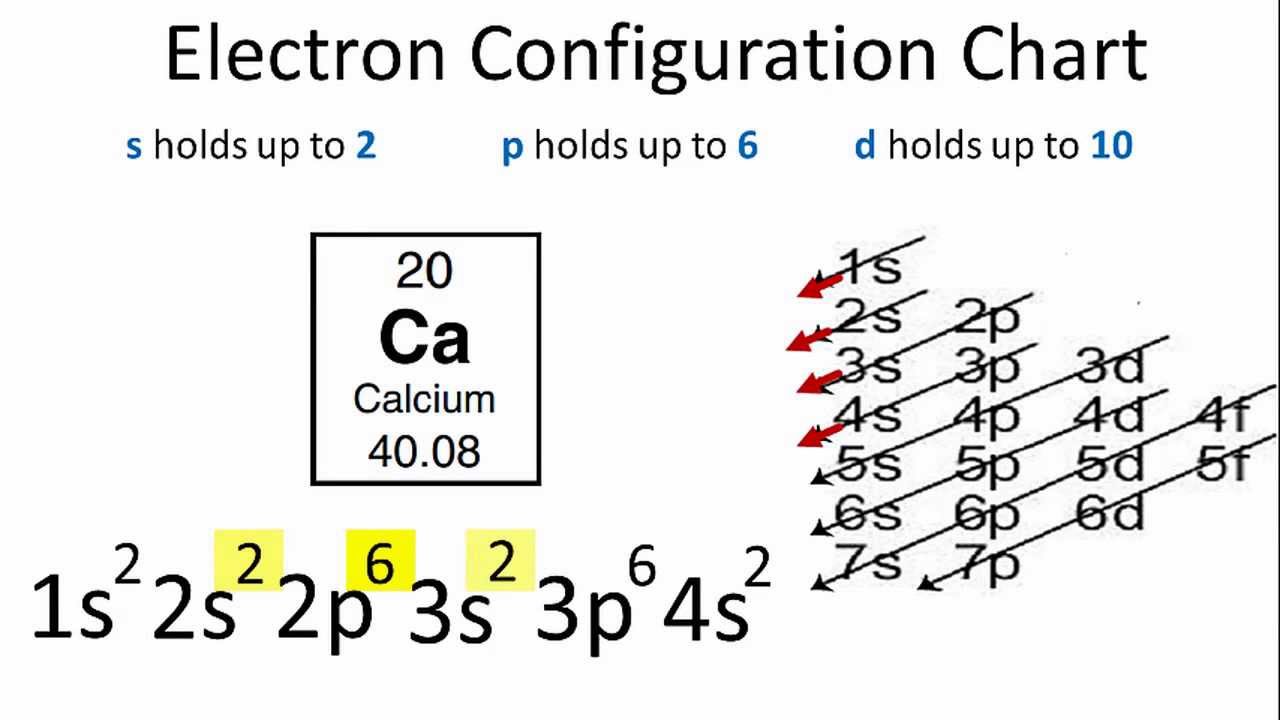
Ground State Electron Configuration for Calcium
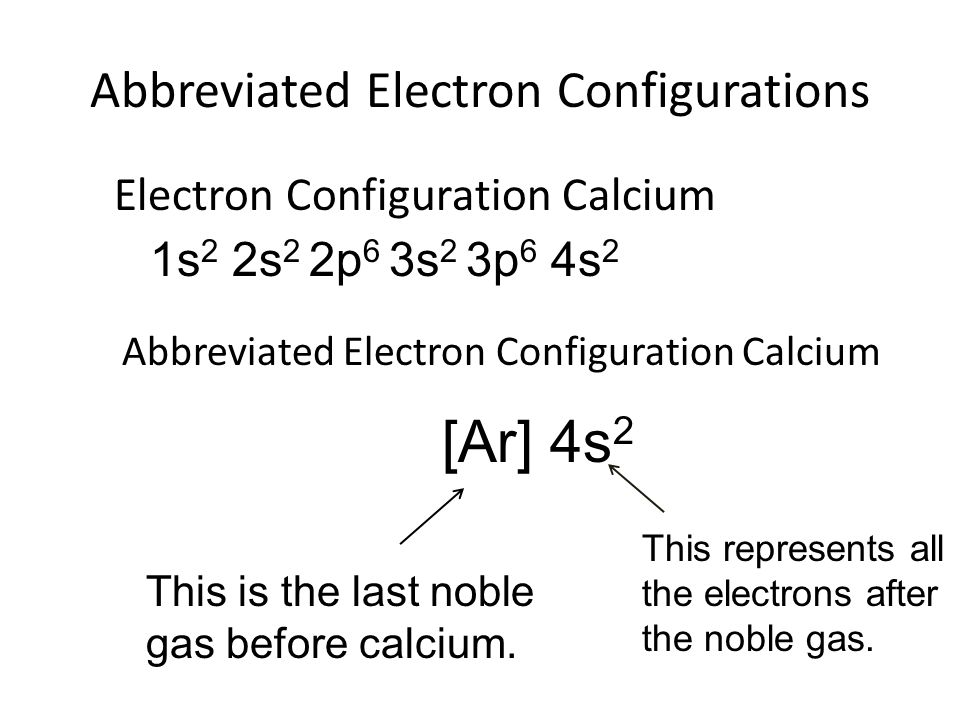
While writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. So Ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2.
How Many Valence Electrons are in Calcium
The calcium has two valence electrons in its outer shell.
What is the Electron Configuration of Calcium
Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. The electron configuration of a Ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2.
Leave a Reply