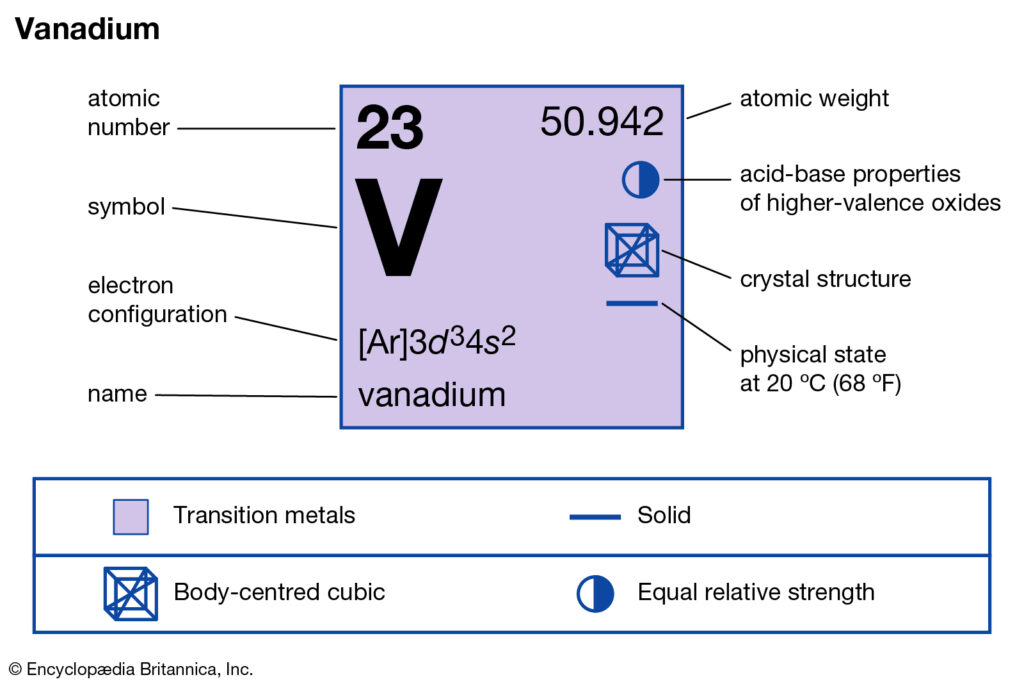
Explore the Vanadium valence Electrons and more about this chemical element. The article would disclose the accurate electron valence of Vanadium. Vanadium is a chemical element that has the very least finding in nature. The chemical element has the atomic number 23 and symbol as V.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
How many valence electrons does vanadium have?
Vanadium in appearance looks like a hard grayish malleable metal. The element is generally produced artificially since it has no proper source in nature. China and Russia are the nations that produce this element in significant quantity. The direct and most use of Vanadium is with the integration of steel.
Many metal industries across the world produce the element in an artificial manner. Furthermore, the tool manufacturing industries use vanadium in the integration of steel to produce tools.
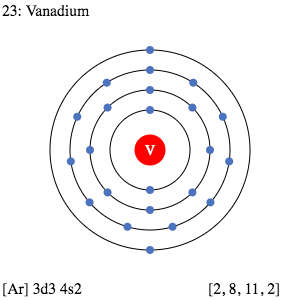
Vanadium Valence Electron Dot Diagram
Well, there are many ways to study the valence electrons of Vanadium. For instance, you can directly check out the periodic table to explore everything about the element. Further, we have the Lewis dot diagram to serve the same purpose. You can use this tool to figure out the valence electrons of atoms within the molecule.
The diagram is very easy to read and understand with the basic knowledge of chemistry. It will guide you in the visualization of valence electrons for the atoms and molecules. You would figure out whether the valence electrons take place in the lone pair or in binding. It’s a must-read diagram for the proper analysis of valence electrons of Vanadium.
Valency of Vanadium
The valency of vanadium may be either 4 or 5. It’s one of those elements which have more than one variable valency. Vanadium is basically a transition chemical element hence it may have variable valency. You may read more about the variable valency of vanadium on the internet.
Leave a Reply