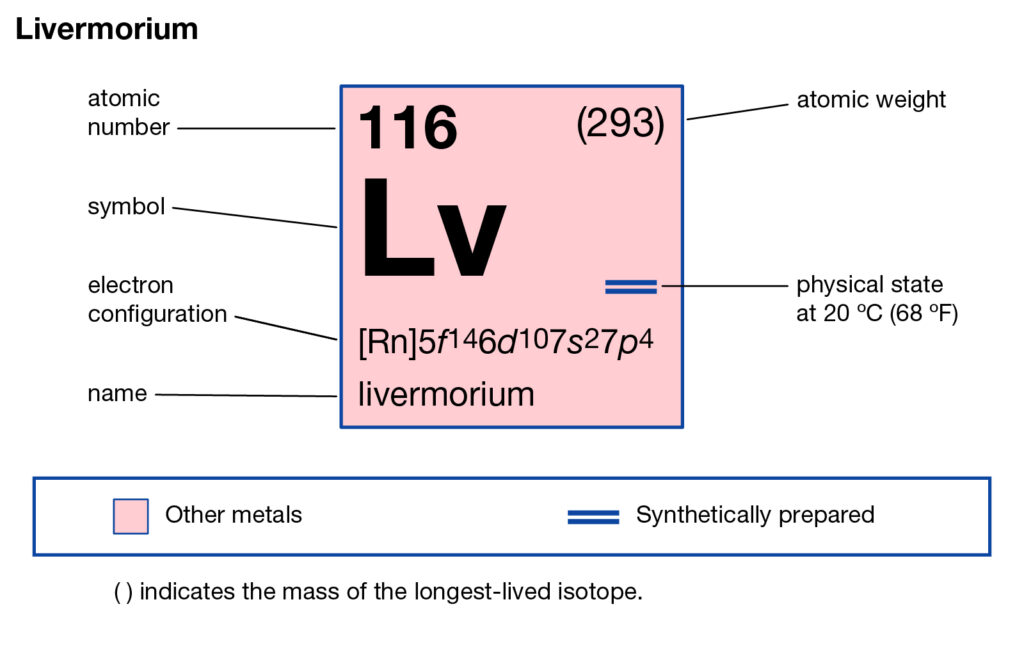
Get to understand the livermorium valence electrons here and enhance your knowledge a step further. The article will help the readers in the systematic learning of this element. Livermorium is purely a synthetic chemical element in chemistry. It has an atomic number as 116 and the symbolic sign of Lv.
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
How many valence electrons does Livermorium have?
The chemical element was first discovered somewhere between the years 2000-20006. It is purely a laboratory-made element and has its roots in Russia. Being the synthetic chemical element Livermorium is a highly radioactive element. It has very high toxic properties and hence not advisable for human exposure.
Livermorium is a highly expensive chemical element due to its less availability. Furthermore, it has short life due to fast decaying properties.
As of now, Livermorium is still in its research purpose hence it has no commercial usage yet. The element is permissible only for research purposes within the laboratory. So, in conclusion, livermorium is a highly radioactive chemical element with nearly no stable isotopes. The element might be useful in nuclear-based researches.
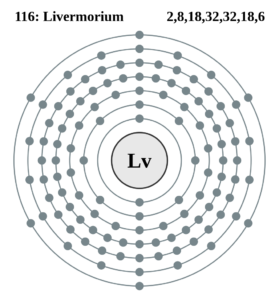
Livermorium Valence Electrons Dot Diagram
Lewis dot diagram is a very famous name in the study of chemical elements. The diagram is useful to represent the numbers of valence electrons for chemical elements. The diagram uses the dots to denote the numbers of valence electrons of atom. It draws the numbers around the symbol of Livermorium.
The numbers of dots are exactly equivalent to the numbers of valence electrons. It also helps in understanding the chemical interaction and then the reaction of valence electrons.
Valency of Livermorium
Well, Liverorium may have the possible variable valencies in different scenarios. It may hence hold the valency of -2,+2 and +4 as well.
So,valency is the combining capability of Livermorium to combine with other elements. It is a very useful bonding property of the given chemical element.