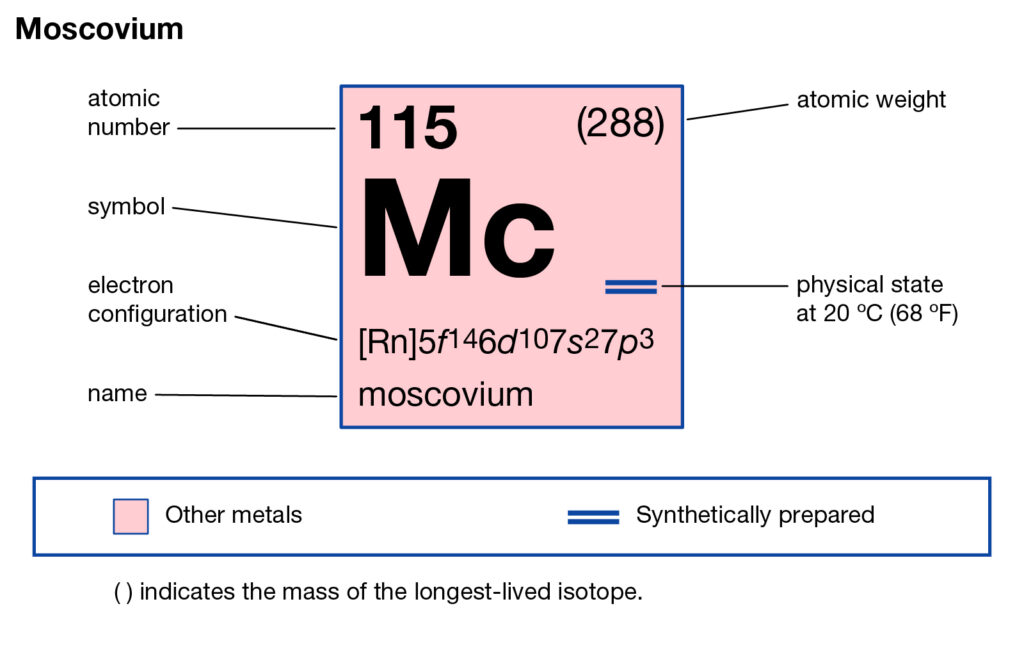
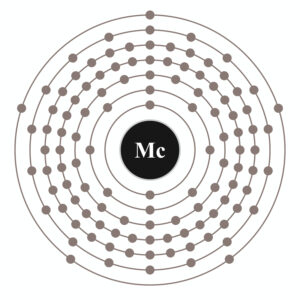
Our science enthusiasts can here study the Moscovium valence electrons. They can further explore the various chemical properties of the element. In chemistry Moscovium is a synthetic chemical element. The element has the symbolic sign as Mc and the atomic number as 115.
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
How many valence electrons does Moscovium have?
The high atomic number of Moscovium conveys about the properties of this element. It is purely a radioactive chemical element. The element was synthesized back in the year of 2003 by Russian and American scientists. The element later got recognition of a chemical element in 2015.
So, Moscovium is one of the recently generated chemical elements. It is highly toxic in nature and hence not advisable for human exposure. Being the synthetic chemical element Moscovium goes through the typical bombarding process to get its form. It has only one stable isotope as Moscovium 290 with a life of less than a second.
Moscovium is still in its research phase hence it has no other known chemical properties of the periodic table. The only known chemical properties of the element are its toxic nature. So, in the present scenario, Moscovium is not available for its usage in the commercial domain. It’s further illegal to use in human exposure since it’s lethal.
Moscovium Valence Electrons Dot Diagram
Lewis dot diagram is probably the best tool to study the Moscovium valence electrons. The diagram makes it easier to study the valence electrons of atoms for the readers.
It further simplifies the interaction of Moscovium valence electrons. The interaction of valence electrons is responsible for the chemical bondings.
Valency of Moscovium
Well, in straight words Moscovium holds the valency of 3. The element hence has the combining capacity as 3 valence electrons.
The combining capacity is responsible to combine with other elements. It’s highly relevant chemical properties of the given element.