Potassium Electron Configuration: Potassium is a chemical element. Its symbol is K that is taken from Neo-Latin kalium. The atomic number of potassium is 19. Firstly it was isolated from potash and the ashes of plants, from where its name is derived. In the periodic table. Potassium (K) is one of the alkali metals.
- H Electron Configuration
- He Electron Configuration
- Li Electron Configuration
- Beryllium Electron Configuration
- Electron Configuration for Boron
- C Electron Configuration
- Fluorine Electron Configuration
- Electron Configuration for Neon
- Vanadium Electron Configuration
- Clorine Electron Configuration
Potassium Electron Configuration
All of the alkali metals have only one valence electron in the outer shell of the electron, which can be easily removed to create an ion of a positive charge – a cation, that forms salts by combining with anions. Potassium occurs only in ionic salts in nature.
The basic elemental potassium is a silvery-white soft alkali metal that oxidizes instantly in the air and reacts vigorously with water. With water, it generates sufficient heat that ignites hydrogen which gets emitted in the reaction and burning with a lilac-coloured flame. Today we are going to tell you about the electron configuration of K.
Electron Configuration for Potassium Ion
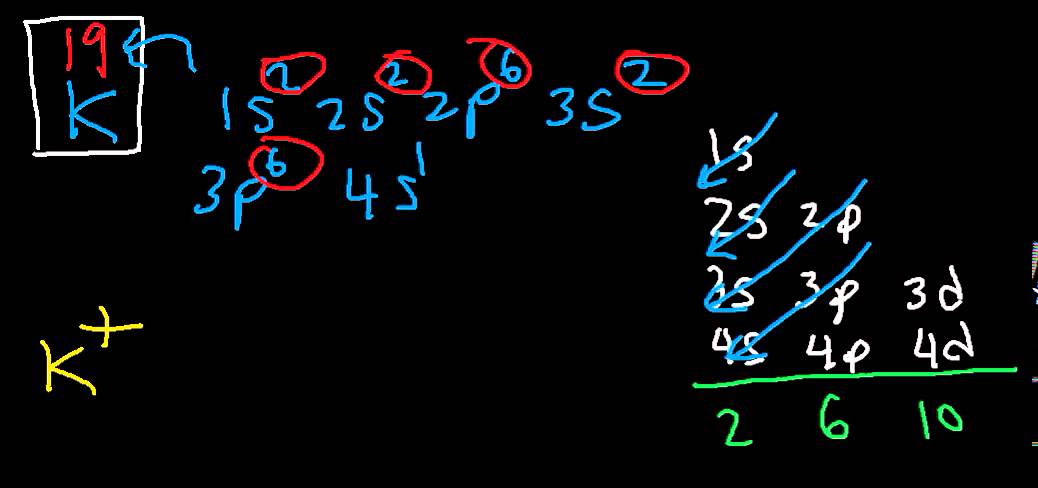
You can see the electron configuration for K Ion in the picture below;
How Many Valence Electrons Are in K?
The potassium Number of Valence Electron is nineteen since Potassium is the alkali metal.
Ground State Electron Configuration for K
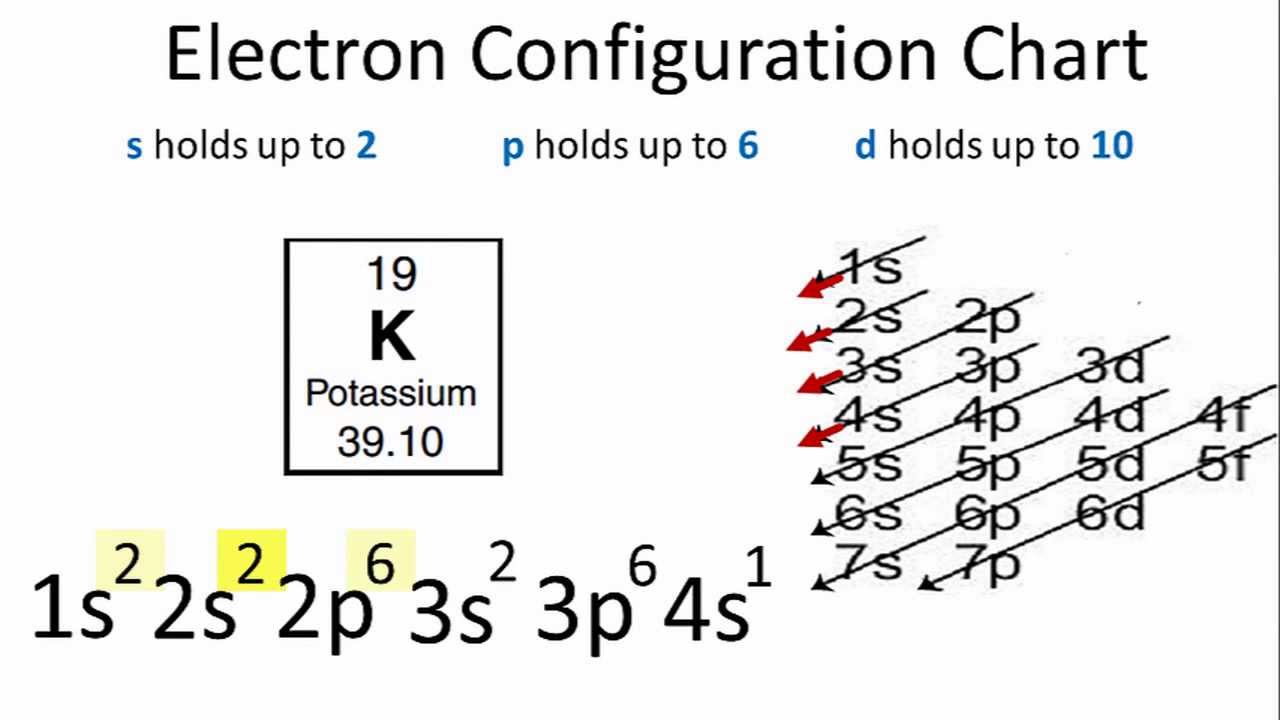
The ground state electron configuration for K is 1s22s22p63s23p64s1
What is the Electron Configuration of Potassium?
The distribution of electrons of an atom or molecule in atomic or molecular orbitals is called the electron configuration, You can find the electron configuration of K in the picture below
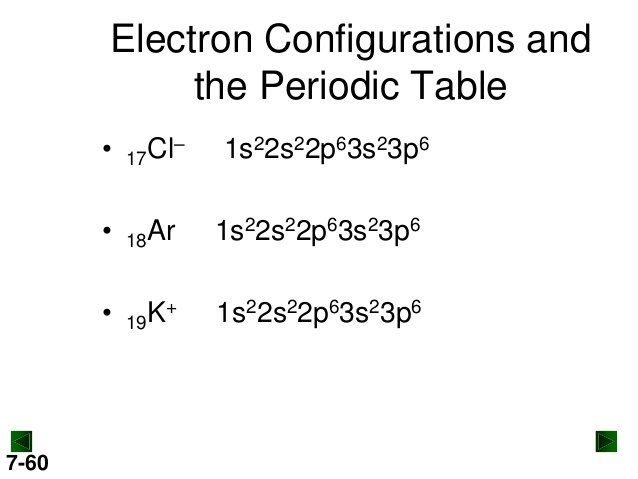
What is the Electron Configuration of K+?
As an ion potassium exists as K+, which means it loses an electron because it needs to attain stable electronic configuration and also the octet valance shell structure which resembles that of noble gases. So as K+, it’s configuration is 2.8.8 without the “extra” 1.