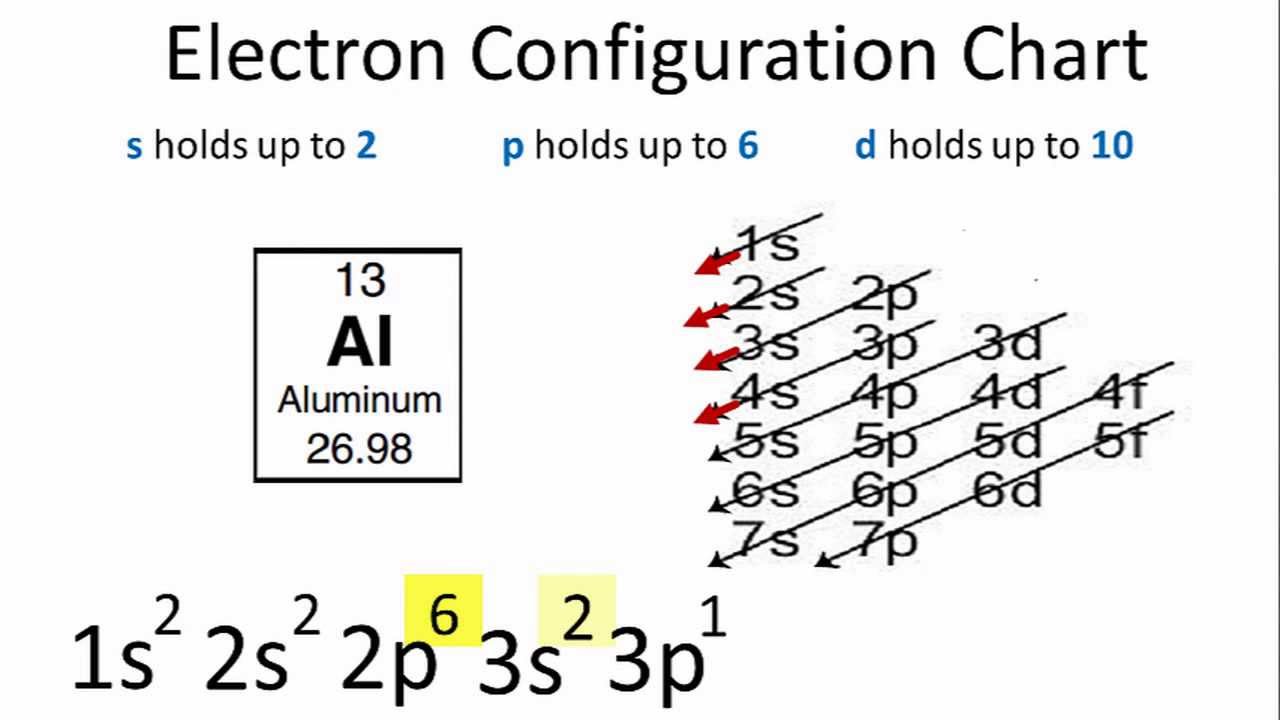
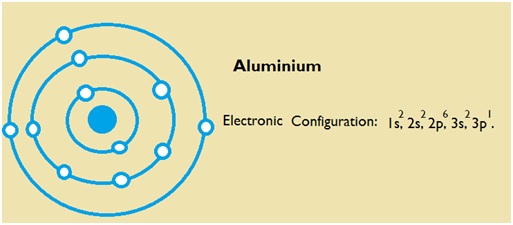
Aluminium Electron Configuration: Chemical element Aluminium can be written as AL and has atomic number 13. Aluminium is a soft, ductile, magnetic and silver-white element which is from the BORON group. Electrons lie in the orbits of atom and molecules and the electron configuration can be termed as the distribution of a number of these electrons in the orbits.
Aluminium Electron Configuration
1s22s22p63s21p1
- Ar Valence Electrons
- Ca Valence Electrons
- Ne Electron Configuration
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Helium Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Carbon Electron Configuration
- Oxygen Electron Configuration
- Aluminum Valency
- Phosphorus Valency
Electron Configuration For Aluminium ion
Electron configuration for Al ion is written in the form: 1s22s22p63s21p1
Aluminium Number of Valence Electrons
As aluminium is a part of a 3rd group and has 3 valence electrons in it.
How Do You Write The Electron Configuration For Aluminium?
To write the electron configuration for Al, it is written on the basis of a number of electrons present in the corresponding orbits and for AL it is written as:
1s22s22p63s21p1
How Many Valence Electrons Are There in Aluminium?
The element aluminium lies in the “13” group also known as 3 or 3A group. It consists of three dots are put to draw the Lewis structure of aluminium.
Electron Configuration For al 3+
Al 3+ means there are 3 electron removals and in the electron configuration, there will be a removal of electrons from the last orbits. And for Al 3+ it can be represented as 1s22s22p6