Electron Configuration For Gold: Gold has the symbol “Au” and one of the naturally occurring higher atomic number elements. In its pure form, the GOLD element is slight, bright, reddish yellow, soft, dense, ductile and malleable metal. Au is a transition element and lies in an 11th group of the periodic table.
- Sodium Electron Configuration
- Germanium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Mercury Electron Configuration
Electron Configuration For Gold
-
Element Symbol: Au
-
Atomic Number: 79
-
Full Configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 5d¹⁰ 6s¹ -
Shorthand Configuration:
[Xe] 4f¹⁴ 5d¹⁰ 6s¹ -
Valence Electrons: 1 (from 6s¹)
-
Unique Property: Relativistic effects make gold appear yellowish
-
Common Oxidation States: +1, +3
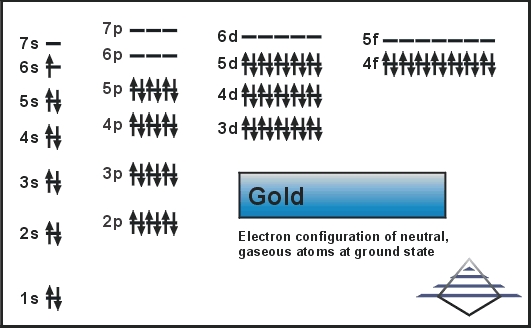
The electron configuration of an element is defined as the number of electrons present in orbits of atom or molecules.
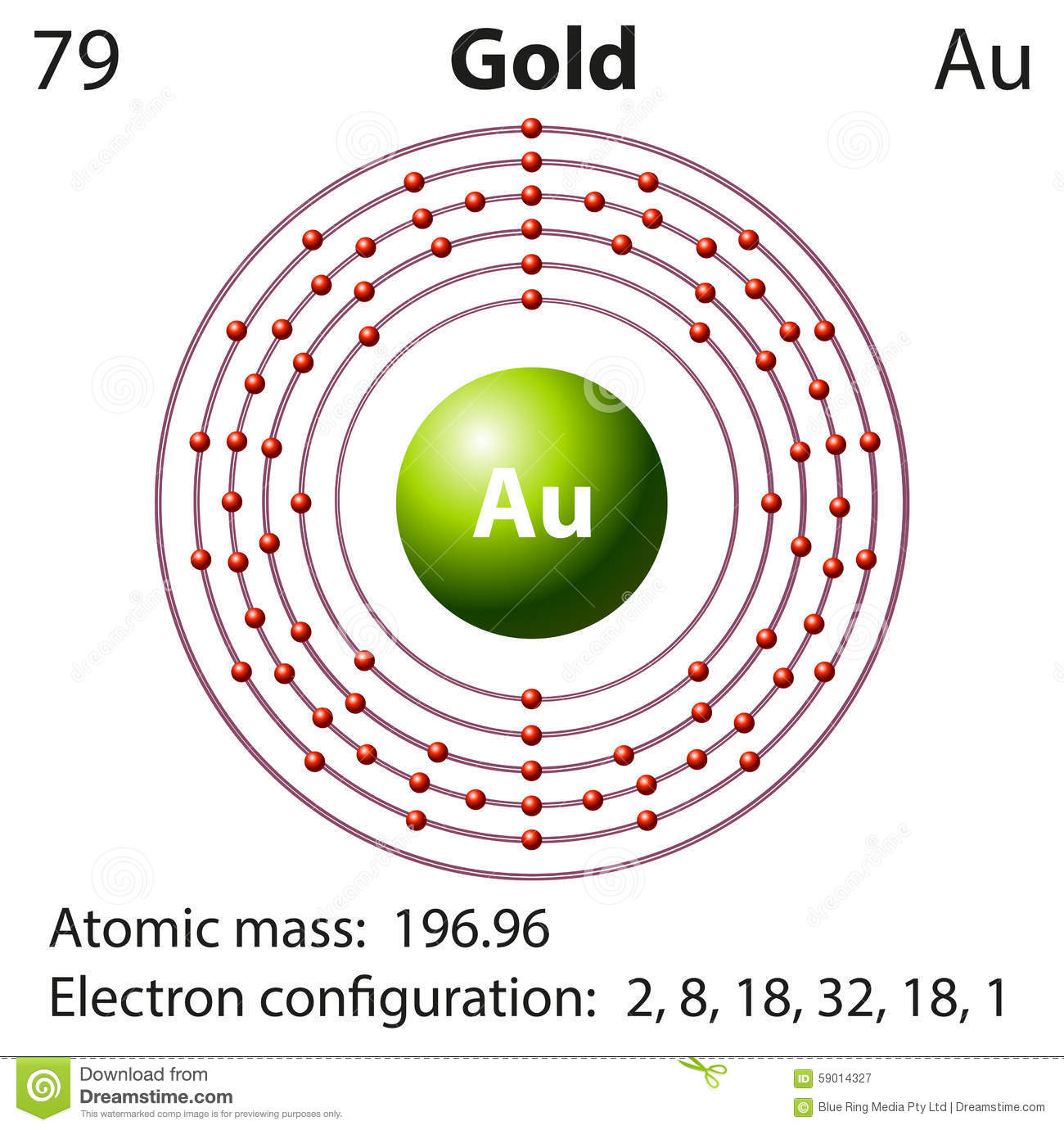
In case of gold, there are 79 electrons present in the 6 shells of the gold atom. The number of electrons in each orbit is 2, 8, 18, 32, 18, and 1. the electronic configuration of Au is:
Chemical Element with atomic number 79.
1s22s22p63s23p63d104s24p64d104f145s25p65d106s1.
Or
[Xe] 4f145d106s1
Electron Configuration For Gold Ion
The electron configuration for Gold ion can be represented as several electrons present in the orbit of Gold Atom shell and it is written as [Xe] 4f145d106s1
Full Electron Configuration For Au
The full electron configuration for Gold can be defined as:
1s22s22p63s23p63d104s24p64d104f145s25p65d106s1
Valence Electrons of Gold
There is only one electron present in the outermost shell of Au so the number of valence electrons Gold have is ONE.
Electron configuration is the distribution of electrons of atoms in the orbits. For Au electron configuration is 1s22s22p63s23p63d104s24p64d104f145s25p65d106s1
How Many Valence Electrons are in Gold
A number of valence electrons are a number of electrons that are present at the outermost shell of the atom. Au has 79 electrons and has only one electron in the outermost shell and it makes univalent compounds. So, the Au has only 1 (one) valence electron.
Leave a Reply