Get to know and study the Lanthanum electron configuration here in our article and explore this chemical element thoroughly. Here in the article, we are going to provide the basic information on this element along with its electron configuration to help our readers in their learning.
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
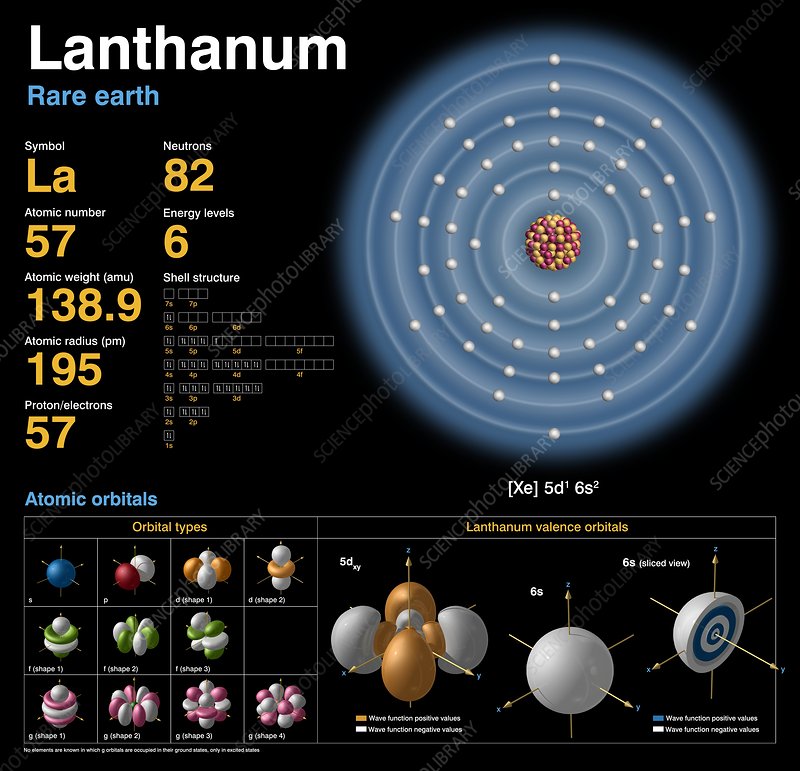
Well, Lanthanum is basically the name of a chemical element that holds its place particularly in the chemistry branch of science. It has the atomic number 57 and the symbol of La for identification purposes. The element belongs to the family of Lanthanide series of elements in the periodic table.
Lanthanum Electron Configuration
It has the physical structure of silvery and white metal which feels soft while touching with the bare hands. If you keep Lanthanum.
In the exposure of the air then it just easily tarnishes as the integral part of its properties. Furthermore, the element also counts itself among one of the rarest earth elements with its oxidation state of +3.
This particular chemical element was first discovered by a Swedish scientist during the year 1839. In the present time, the element occurs in nature with the cerium and such other rare chemical elements. So, basically, one thing is clear with the nature of this element that it has no free form.
Being one of the rarest chemical elements it also has very limited availability in nature. However, in the category of Lanthanide chemical elements, it is considered to be one of the most abundant chemical elements.
Electron Configuration For La
Well, we believe that the electron configuration of this chemical element is one of the most significant parts of its properties. This is the reason that why scholars mostly concern about the electron configuration of Lanthanum.
So, we basically have the Lanthanum electron configuration is [Xe] 5d¹ 6s² in its standard form. Kindly note that this is the standard abbreviated form of the electron configuration for Lanthanum and you can easily derive it by using the standard formula for the same.
How Many Valence Electrons Does Lanthanum Have?
So, the electron configuration is essential since this is the process in which Lanthanum basically distributes its electrons to the atomic orbitals of the element. This process as a result provides an equation or the denotation that we write as the electron configuration. Here below are some of the significant aspects of the Lanthanum electron configuration.
- Lanthanum electron configuration is useful in finding out the valency of the element.
- The electron configuration also decides the nature and place of the element in the periodic table.
- It breaks out the whole element and finds its other potential users.
- The reaction properties of the element are also known with the help of its electron configuration.
So, all these aspects combined make the electron configuration quite significant for the chemical element.
Well, Lanthanum is one of those chemical elements that have very diverse usage in the industrial domain. This is what makes it quite a demanded element. Some of the popularly known usages of the element include such as a gas mantle, alloys production, arc lamps, etc. There is particularly a long list of applications of this element in the various industries and it is even useful in some medications as well. It however has no biological role in human exposure and is not very toxic to humans. We hope that this study of Lanthanum electron configuration will help our readers to understand the element in a good manner.
Leave a Reply