Explore the chemical element of Copernicium valence electrons and get to know the element from a close dimension. Here in the article, we are going to make our discussion on the valence electrons and the valency of the Copernicium. As a scholar of chemistry, you will be able to develop a better understanding of this chemical element with the information provided in the article.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
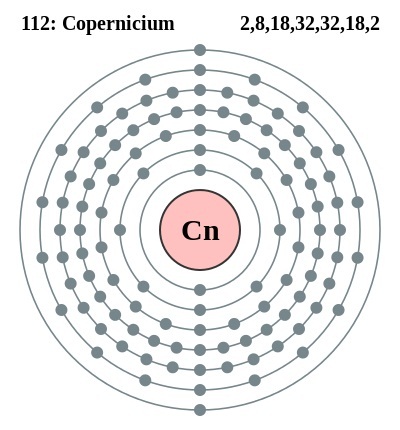
Copernicium is the chemical element that comes from the family of synthetic elements in the periodic table. The element carries its atomic number as 112 and the symbol of Cn. Being the synthetic chemical element it mostly has highly radioactive isotopes. For the same reason, the element is not safe for human exposure and is only available for research purposes within the laboratory.
How Many Valence Electrons Does Copernicium Have?
The valence electron is the most significant aspect that forms the integral properties of this element. Valence electrons are basically the numbers of the electrons that are present in the outer shell of Copernicium for the atoms. These electrons take a direct part in the chemical bonding process of the element. Subsequently, the valence electrons form part of Copernicium’s actual properties. With the proper information of the valence electrons, one can figure out that whether the element is capable of bonding with the other elements. It also provides the exact numbers of the elements with whom it can actually have its bonding.
Copernicium Valence Electrons Dot Diagram
Well, there are a number of ways to study the valence electrons of the Copernicium and the electrons dot diagram is one of those methods. This method is also well famous as the Lewis dot diagram in the domain of chemistry. The diagram basically uses the dots as the numbers of electrons for the chemical element. The diagram shows that whether the electrons lie in the pair or as the sole units in the outer atom molecule of Copernicium.
Valency of Copernicium
The valency is also the aspect of the chemical properties of the element that comes by solving the valence electrons equation. We also term the valency as the combining capacity of Copernicium. So, Copernicium basically has the valency of 2 which is relevant in the chemical bonding of the element.