Learn about the Roentgenium valence electrons and explore this chemical element in a thorough manner. Here in the article, we are going to cover up the much useful information on the valence electrons and the valency of the Roentgenium for all our chemistry scholars.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
How Many Valence Electrons Does Roentgenium Have?
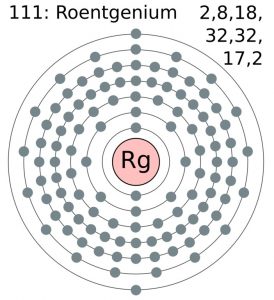
It’s basically the chemical element and is located in the periodic table in its specific position. Roentgenium has the atomic number 111 and the symbol of Rg. The element belongs to the category of synthetic elements that are created primarily in the laboratory only. Subsequently, the Roentgenium holds extreme radioactive properties and is not advisable for human exposure. The element got its birth in the year 1994 in the laboratory itself and is still in its research phase.
Well, the valence electrons of a chemical element basically refer to the number of electrons in the outer shell of the element for the atom. So, basically, Roentgenium has the valence electrons as 6d10 7s1 as per its periodic table. The role of the valence electron comes in the formation of the chemical bonding of the element with other elements. It indirectly lets the chemist know that whether the element will establish its binding with the others or not. It can also tell the readiness of the bonding and the number of bonding.
Roentgenium Valence Electrons Dot Diagram
Dot diagram can prove to be quite significant in the visualization of the valence electrons of an atom for the element Roentgenium. The dot structure diagram has been given by Lewis and it reveals the actual number of valence electrons. It also reveals that whether the valence electrons take place in a pair or in lone within the molecules. So, we highly recommend our chemistry scholars go through the dot diagram for a better understanding of valence electrons.
Valency of Roentgenium
The valency of Roentgenium is the combining capacity of the element with the other elements. It actually refers to the number of electrons that it may gain or lose to attain its stable form of electron configuration. Roentgenium has the precise valency of 3 which is an integral part of its properties.