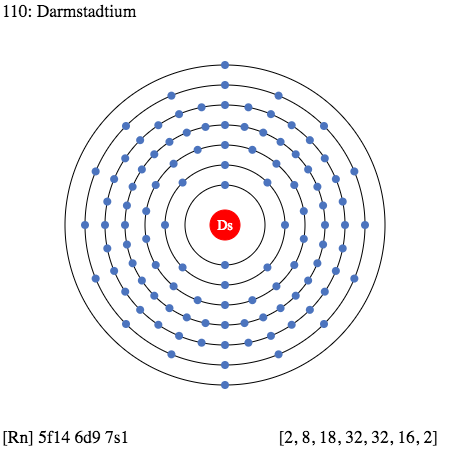
All our scholars of chemistry can here explore the Darmstadtium valence electrons and explore the other useful properties of the element. The article shed some light on the electron configuration and the valency aspect of this chemical element. It provides a useful study on the various aspects of Darmstadtium to assist the scholars in their systematic study of the element.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
Darmstadtium is basically the chemical element that is part of the periodic table in the study of chemistry. It has the atomic number 110 and the symbol of Ds for representation and identification purposes. The element is purely synthetic in its composition and therefore has high radioactive properties. The element formed its birth in the year 1994 within the lab by a German scientist. Presently the element is available only in its research phase and has no mainstream usage.
How Many Valence Electrons Does Darmstadtium Have?
In the periodic table of chemistry, the Darmstadtium belongs to the category of the transition metals which have the characteristics of having the variable valence electrons. So, with the same theory, the element Darmstadtium can have valence electrons between the numbers of 3 to 12.
You should be aware that the valence electron is basically the number of electrons that are located in the outer shell of the element. The valence electrons have a significant role to play while having the bonding with the other chemical elements. These are the significant properties of the element which are quite useful in the research phase of this element.
Darmstadtium Valence Electrons Dot Diagram
The dot or the lewis dot diagram is the best source for the visualization of valence electrons of the atoms for the element. This dot pattern is built by Lewis in order to provide scholars with better visualization of the valence electrons. It even goes to the extent of providing the information of whether the valence electron occurs in the pair or in the lone bonding. So, with the dot diagram, you can get to understand the Darmstadtium valence electrons in a better way.
Valency of Darmstadtium
The exact and accurate valency of Darmstadtium is 6 as per the periodic table. We also know valency as the combining capacity of the element with the other elements. Each and every element has its own valency that assists in the better analysis of the element.