Beryllium Valence Electrons: Beryllium is symbolled as (be). It is a chemical element that is the lightest member of alkaline earth metals. Beryllium is a type of steel-grey color metal that stays at room temperature. Its chemical properties sort of resembling as aluminum. It has a high melting point and has a very high modulus of elasticity.
- Flerovium Valence Electrons
- Mercury Valence electrons
- Moscovium Valence Electrons
- Bismuth Valence electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Oganesson Valence Electrons
- Tellurium Valence Electrons
- Nobelium Valence Electrons
- Xenon Valence Electrons
- Neptunium Valence Electrons
- Cesium valence electrons
- Plutonium Valence Electrons
- Iodine Valence Electrons
- Americium Valence Electrons
- Radium Valence Electrons
- Gold Valence electrons
- Lead Valence electrons
Beryllium stability and ability are to take a high polish which is useful for mirrors, camera shutters, and semiconductor manufacturing. Beryllium is generally colorless and sweet in taste. The people who work with beryllium could lead to berylliosis which is described as a decrease lung capacity and other similar effects.
How many valence electrons does Beryllium have?
Beryllium is often found in the mineral’s beryl and bertrandite. And this is found in the earth’s crust and igneous rocks. Mostly, beryllium is extracted from the U.S. and Russia.
Beryllium Valence Electrons Dot Diagram
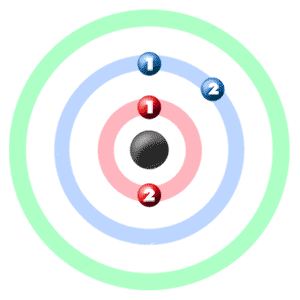
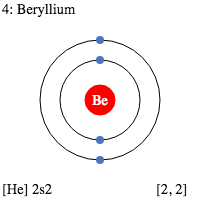
Electron Dot Diagrams are the diagrams where the valency of electrons of an atom is shown as a dot which is distributed around the element symbol. The beryllium atom has two valence electrons and would have an electron diagram as (.Be.). As electrons here repel each other and the dots of an atom will distribute equally around the symbol before getting paired. Here, given the diagram to understand the concept of the valence electrons dot diagram in a very easiest way. And you would definitely understand it and learn it by seeing it.
Valency of Beryllium
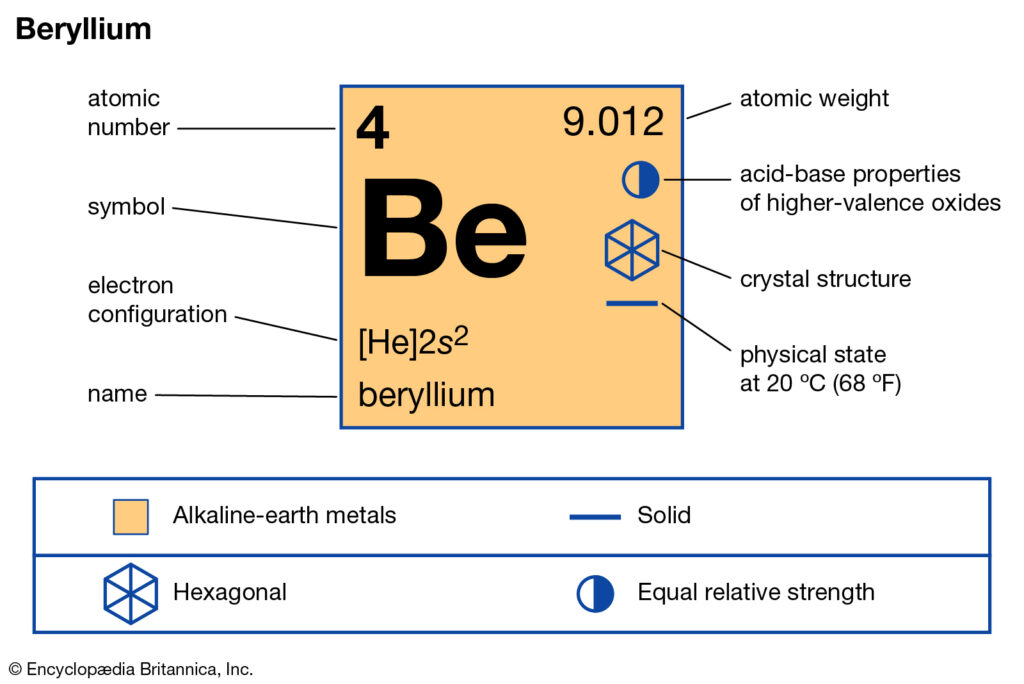
We already know the beryllium atomic number is 4. So, the electronic configuration of Be will be 1s² 2s². And so, its valency will be 2 because it has 2 electrons in its outermost shell. This is how because 2 valence electrons are located in the 2s subshell. And these electrons give a +2 oxidation and their ability is to form the 2 covalent bonds.