Promethium electron configuration is available here in the article for all those chemistry scholars who want to study this element. The article provides the basic information of the electron configuration for the element along with its other chemical properties.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
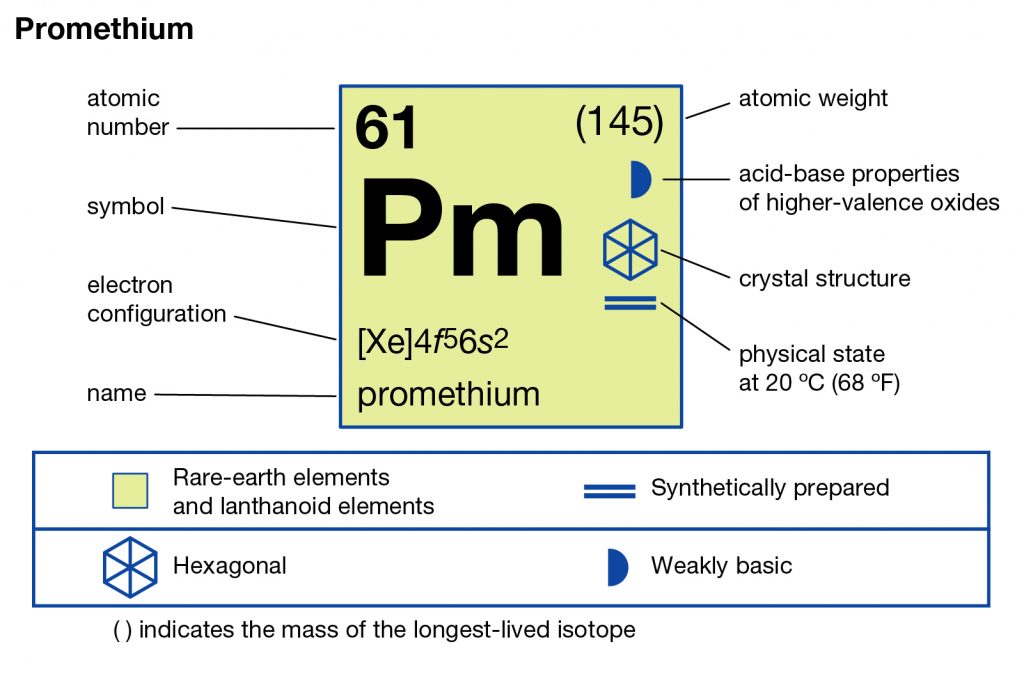
In chemistry, Promethium is the chemical element that comes with the atomic number 61 and the symbol of Pm in the Periodic table. It’s basically a highly reactive radioactive chemical element that is extremely rare in the crust of the earth. The element is so rare that it has the availability of only around 600 grams maximum in the earth’s crust.
How Many Valence Electrons Does Promethium Have?
This fact makes it the super expensive chemical element that has all the radioactive isotopes. Technically the element belongs to the category of the Lanthanide elements and has the oxidation state of +3. The element is basically the member of the period 6 and f block in the periodic table.
Promethium was first discovered in the year of 1902 by a scientist of the Czech Republic. The element showed the characteristics that are most similar to the Neodymium and Samarium chemical elements. In fact, both of these elements are closely related to the Promethium even in the modern age.
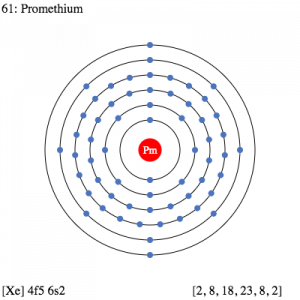
Promethium Electron Configuration
The electron configuration of this chemical element basically defines the process of the distribution of its electrons to the atomic orbitals. This process is denoted in the form of an equation and we know it as the electron configuration for such element. This is basically the integral process that happens with all of the chemical elements in chemistry. Each and every chemical element has its own unique electron configuration.
So, the Promethium electron configuration can be basically written or represented as the Xe 4f5 6s2 in its standard form. This is the short abbreviated form for the electron configuration of Promethium which we get after applying the formula for the same.
Here below are some of the key significances of the Promethium electron configuration.
- The element is extremely useful in the calculation of the valency for the element.
- We can also check out the reaction element of the Promethium by using its electron configuration.
- The element is further useful in determining the other significant properties of the element and its usage on a similar basis.
Electron Configuration For Pm
Well, as we have discussed already in the article that Promethium is basically a radioactive chemical element. The element is highly hazardous for the same cause and is mainly in its research phase. There is the other form of the element as Prompt 147 which has some quite significant usages in the laboratories. The prime form of the element has very high radiation and is useful in the radiation batteries with its natural properties. The element can prove to be the game-changer in its long-term nuclear energy context.
The other possible future usage of the element lies in the portable sources of x-ray, the element of power or heat source in the space energy, etc. The element is very promising in itself and is still in the early research phase. We hope the article would be helpful in providing the Promethium electron configuration and the other properties to all the enthusiasts.
Leave a Reply