Neon valence electrons: Neon is a chemical element that has a symbol ‘N’ and the atomic number of the Neon is 10. Neon is a kind of Nobel gas. It is colorless and odorless gas in nature. Neon has some unique kind of reddish-orange glow. Neon cannot react with any other substance. Nen comes in the group 18 (Nobel gases) of the periodic table.
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Nobelium Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Gold Valence electrons
- Mercury Valence electrons
- Lead Valence electrons
- Bismuth Valence electrons
- Radon Valence electrons
- Radium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium valence electrons
The atomic weight of the neon is 20.1797. The boiling point of the neon is 27.07 K and melting point is 24.56 K. Its room temperature is gaseous in form. Neon is always used in making high voltage indicators. Neon has a non-toxic biological role. It is the fifth element is the whole universe. Neon gives a kind of that friction that contains both compounds which is helium and neon. This is one of the 2nds lightest in all inert gases and has three stable isotopes.
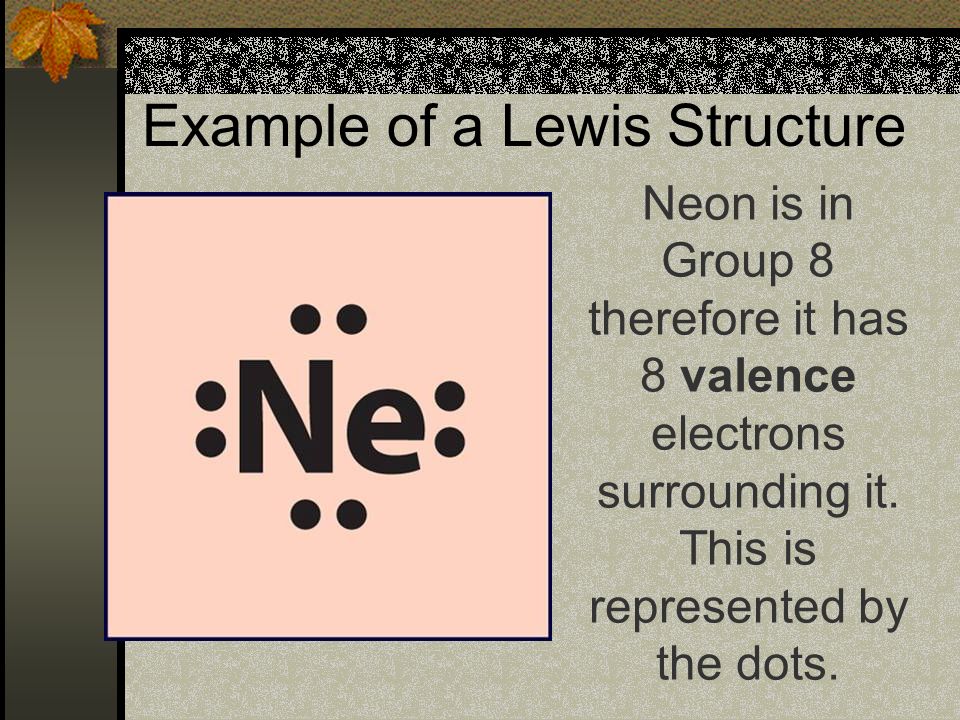
Neon Valence Electrons Dot Diagram
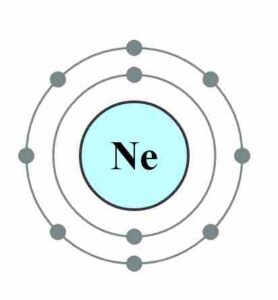
The electronic configuration of neon is 1s2 2s2p6. The valence electrons dot diagram is a diagram where valence electrons of atoms are located in a form of dots around the shells of the atom. The number of valence electrons can be finding out with the help of the electron configuration. We have provided a dot diagram here of the neon chemical element. You can download the table and the diagram of the neon and also of the other elements in the periodic table. You can anytime visit to our website and check out the relatable details about the elements and their valences.
Valency of Neon
Neon is an element that belongs to the noble gas. Its atomic number is 10. Neon has eight valence electrons. In the diagram, the 2s orbit has 2 valence electrons where the 2p orbit has other six valence electrons, which makes a full octet of 8 valence electrons, which makes the shell satisfied and stable. And, in this way, the valency of Neon is 0.
Leave a Reply