Sodium Electron Configuration: The chemical element sodium has the symbol Na and atomic number 11. This is soft, reactive, silver + whitish metal. Electron configuration can define as the distribution of electrons of molecules or atoms in molecular or atomic orbits.
- Phosphorus Valence Electrons
- S Valence Electrons
- Cl Valence Electrons
- Ar Valence Electrons
- C Electron Configuration
- Neon Electron Configuration
- V Electron Configuration
- Potassium Valence Electrons
- Ca Valence Electrons
- Titanium Valence Electrons
Sodium Electron Configuration
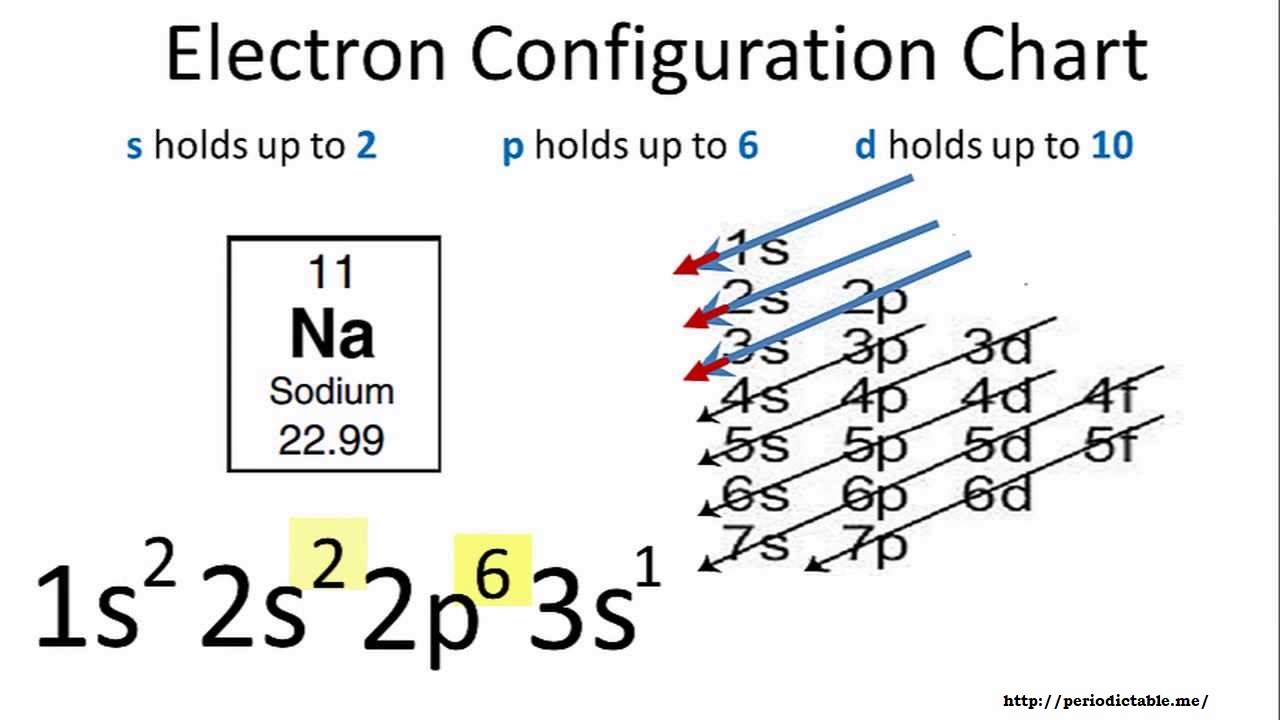
The electron configuration of Sodium can be written as:
1s22s22p63s1
The symbol of Sodium is Na as you can see in the given picture. The number of protons, electrons, and neutrons also mentioned there.
Electron Configuration For Na Ion
If we talk about an electronic configuration of sodium ion then it can write this:
[Ne] 3s1
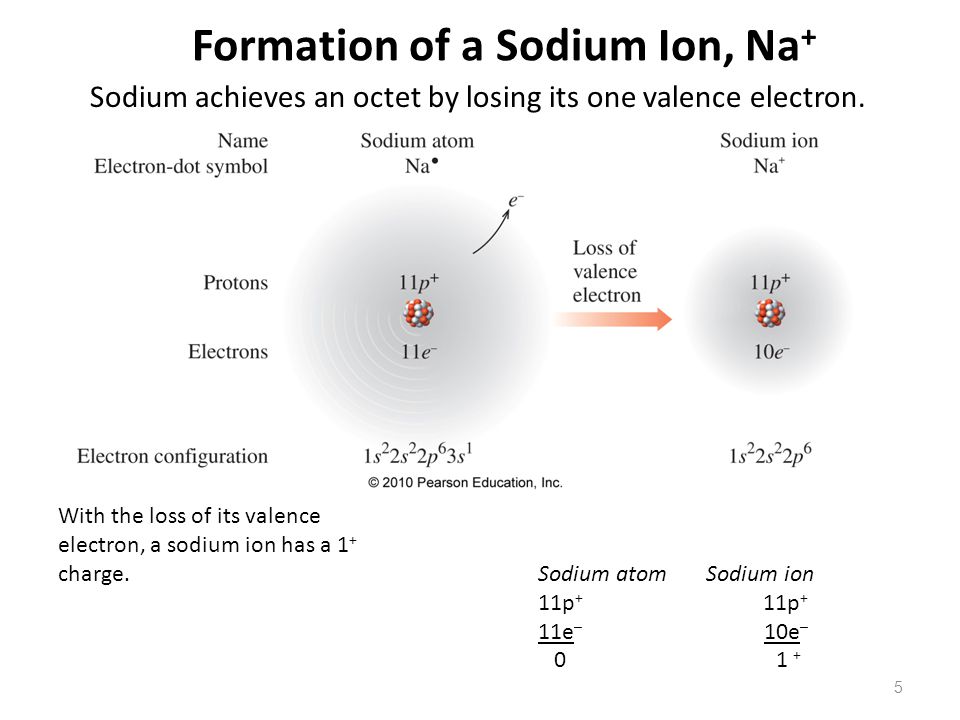
Unabbreviated Electron Configuration For Na
The unabbreviated electron configuration for Na can be represented as:
1s22s22p63s1
How Do You Find The Electron Configuration For Sodium?
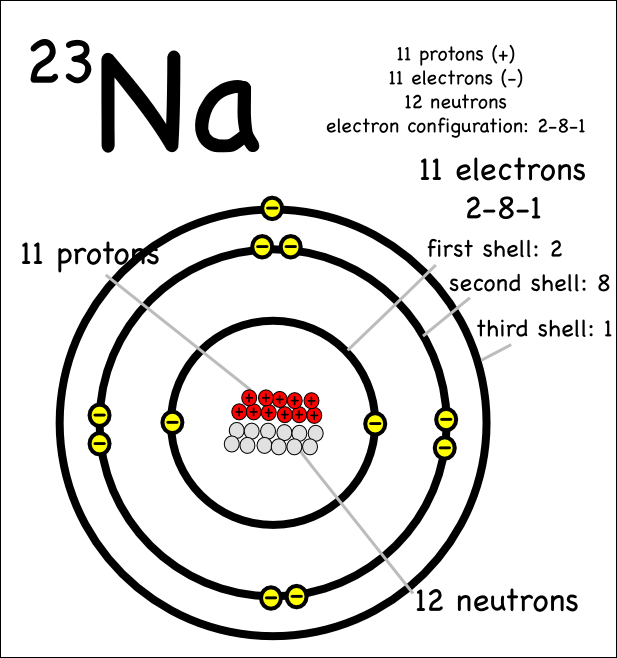
To find the electron configuration firstly you should be aware of the number of electron in the element. The sodium has 11 numbers of electrons. The orbital arrangement of atoms consists of first orbit 1s which can take only 2 electrons after this 2s orbit consists of 2 electrons. You can check the position of Na on the periodic table homepage article.
Then 2p orbit can hold 6 electrons. The remaining 1 electron of sodium shifts to the 3s orbit. In such a way you can find the electron configuration of Na.
How Many Electrons Are in the p Orbital of Sodium?
P orbit can consists of up to 6 electrons so 6 electrons are held by the 2p orbit of sodium.
How Many Protons and electrons are in sodium?
Mostly all elements have the same number of electrons and protons and in the case of sodium, there are 11 numbers of protons and electrons.
Leave a Reply