Learn the basics of the Cerium electron configuration and the other significant properties of the element. Here in the article, we are going to cover up the basic information on this chemical element
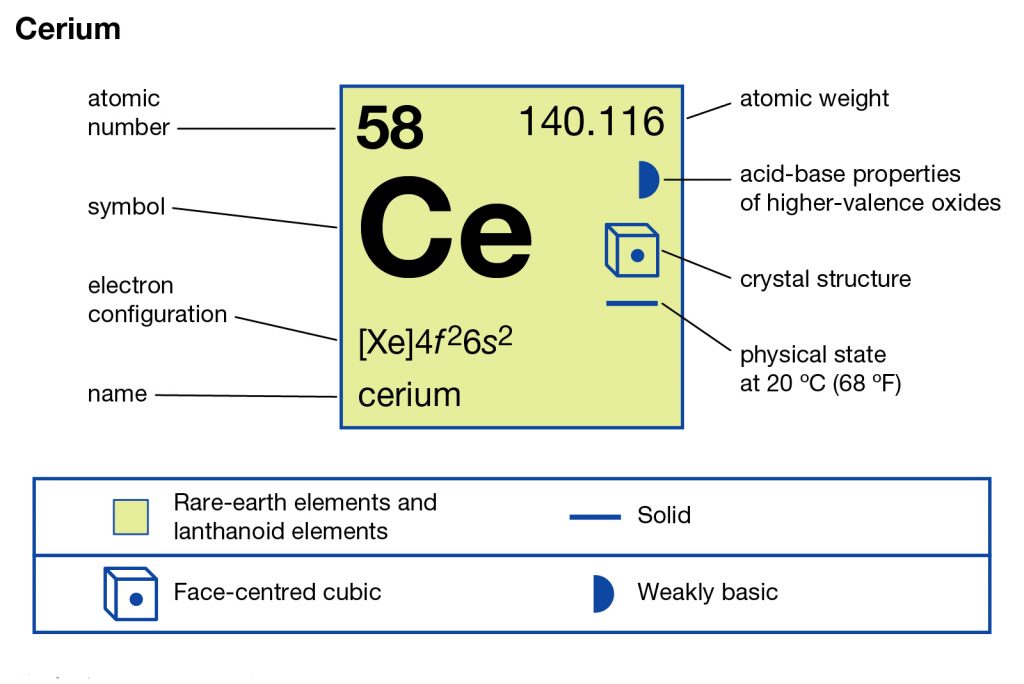
In the chemistry branch of science, Cerium is the name of a well-known chemical element. It has the atomic number 58 and the symbol of Ce as its identity proof. The element has a white silvery and ductile physical structure in its appearance.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
Electron Configuration For Cerium
If you touch this element you will feel a very soft touch of the element that has decaying and tarnishing properties. This is the reason that why Cerium gradually tarnishes in the exposure of air. The element belongs to the category of Lanthanide elements and shows similar properties. You can literally cut the element with a regular kitchen knife in its tarnishing state. It has an oxidation state of +3.
Cerium has no free form of occurrence in nature rather it takes place from some other chemical elements and the ores. It includes the mineral ores such as monazite or bastnasite that are easily available in a number of countries around the world. The cerium basically takes its birth from these ores during the extraction process of the same.
Furthermore, Cerium is one of the rarest earth chemical elements with its limited availability. This is what makes it quite an expensive chemical element in itself with significant demand. There are a number of countries where you can find the Cerium in some limited quantities.
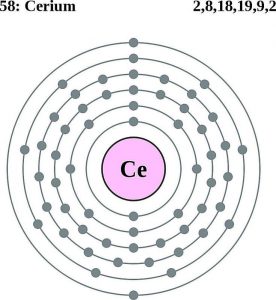
Cerium Electron Configuration
Well, the Cerium electron configuration is a significant part of the Cerium’s chemical properties. You can’t really understand the element without exploring its electron configuration. So for the same reason, all the scholars of chemistry must be aware of the electron conflict for the element.
So, we basically have the Cerium electron configuration is [Xe] 4f¹ 5d¹ 6s² in the short form for the understanding of our readers. We get this abbreviated form of the electron configuration after applying the standard formula for the same. You need to understand that the electron configuration of Cerium is basically the representation of the electron distribution of the element.
This distribution is basically made to the atomic orbitals of the element and then we consequently get the equation of its electron configuration. The electron configuration is significant for the number of factors for such an element. Here below we are dropping down some of the most significant aspects of the electron configuration of an element.
- It helps in calculating the valency of the element.
- The chemical reaction of the element is also determined with the help of its electron configuration.
- Electron configuration is essential in the overall breakdown of the element to find its other properties.
So, all these aspects make the electron configuration study very relevant for all the scholars of the element.
How Many Valence Electrons Does Cerium Have?
Well, as we know that each and every chemical element has its own significance in science. In a similar manner Cerium is also the significant element for all its applications. There are a number of places where the element is considered to be very useful. You can see its applications in the gas mantles, alloys production, polishing compound, led production, several medications, etc.
So, these are some of the most common usages of the Cerium in its industrial context. There are many such other minor and significant usage of the element in the other prominent places as well. We hope that with this article our readers would be able to study the Cerium electron configuration in an appropriate manner.
Leave a Reply