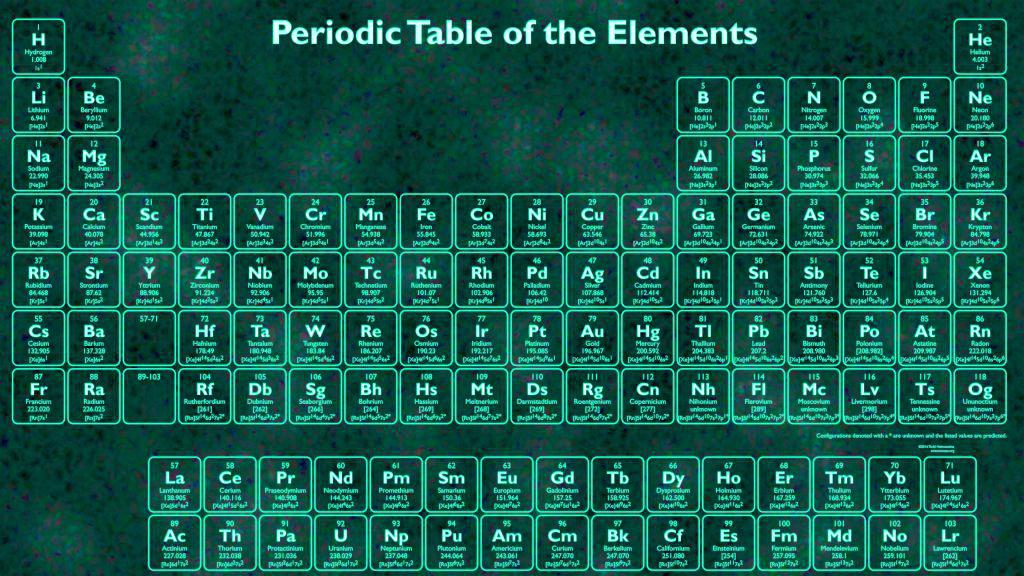
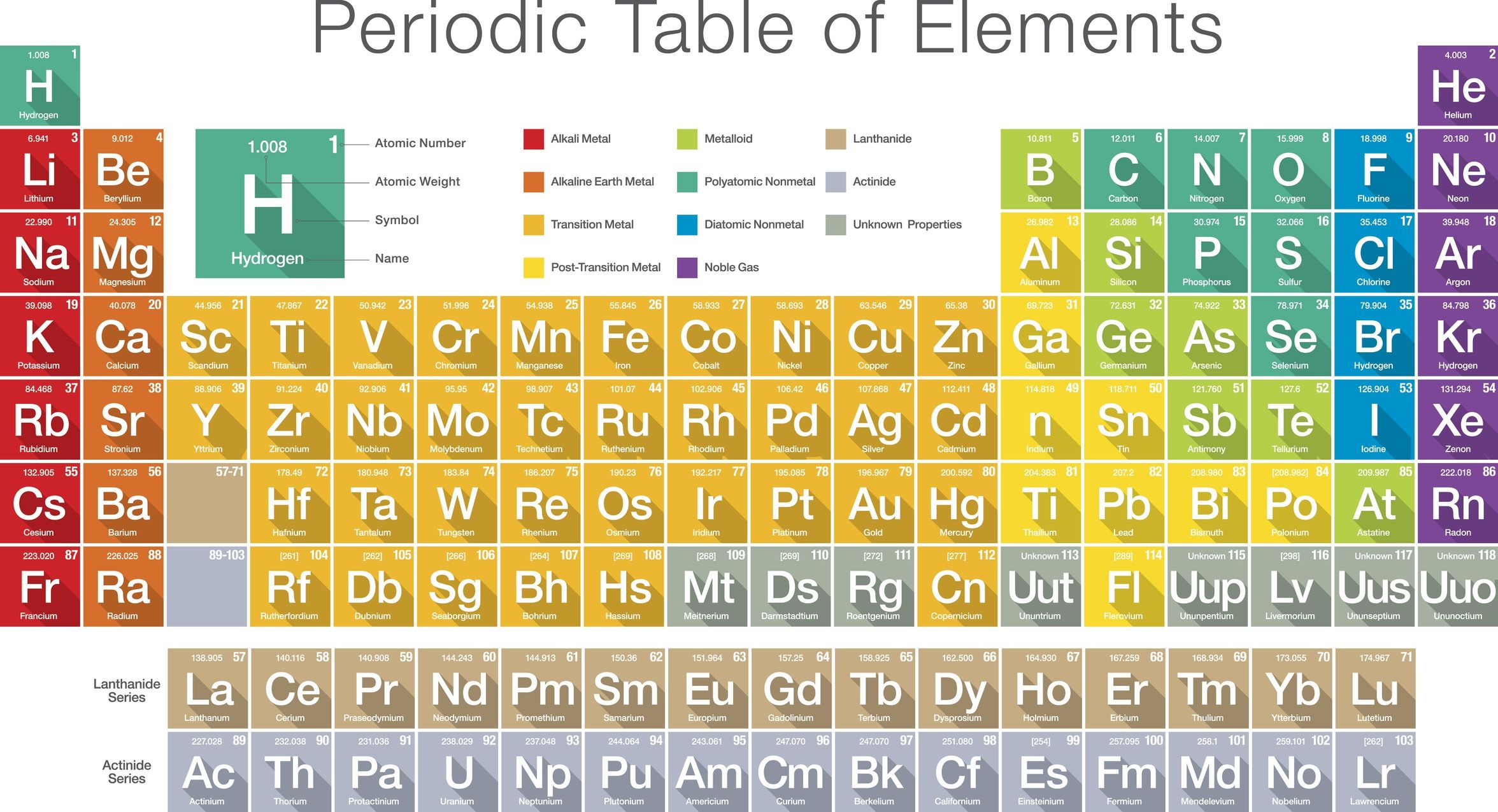
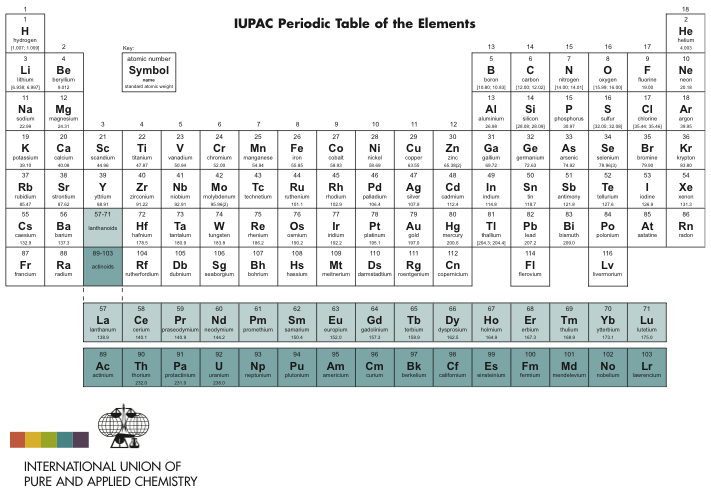
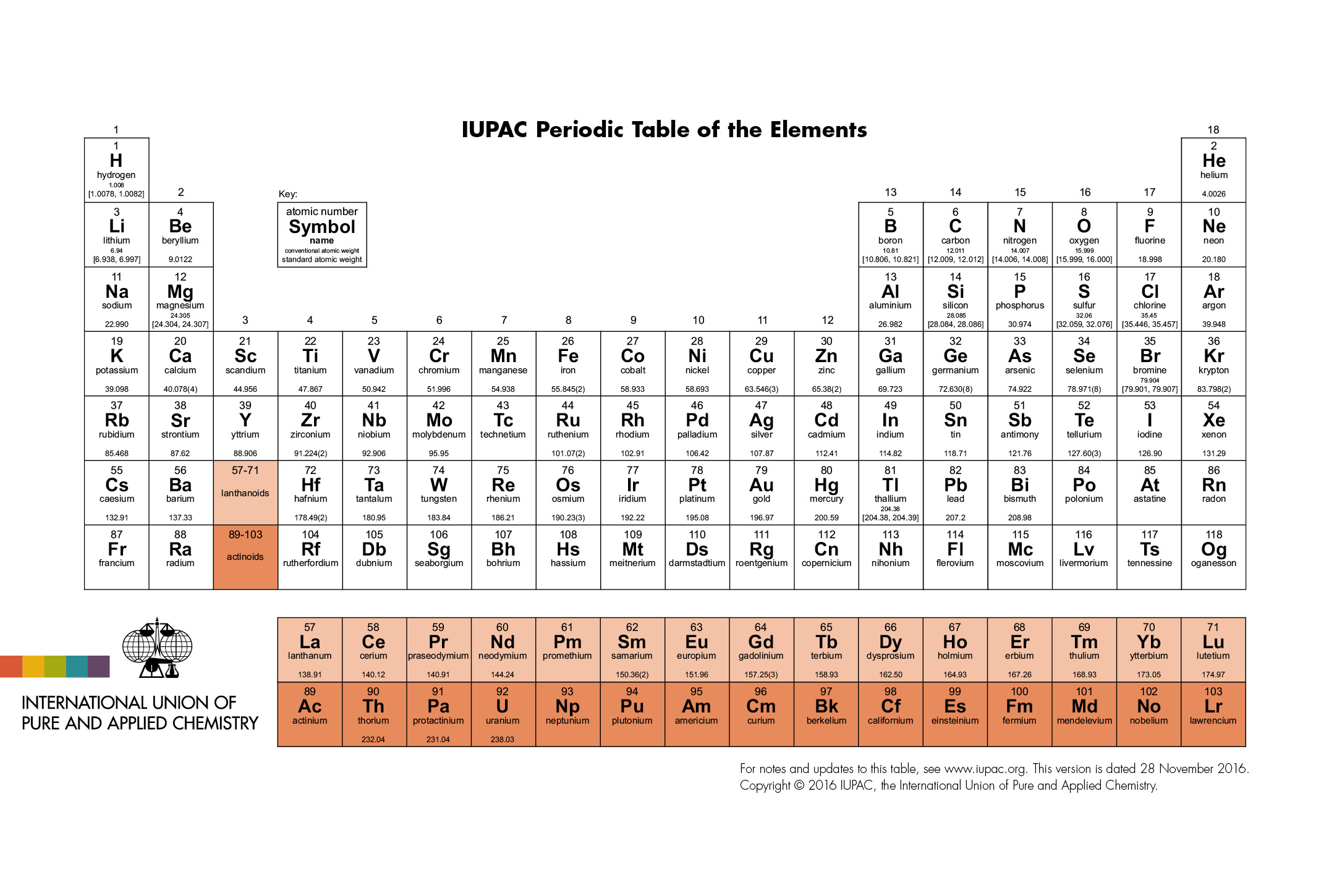
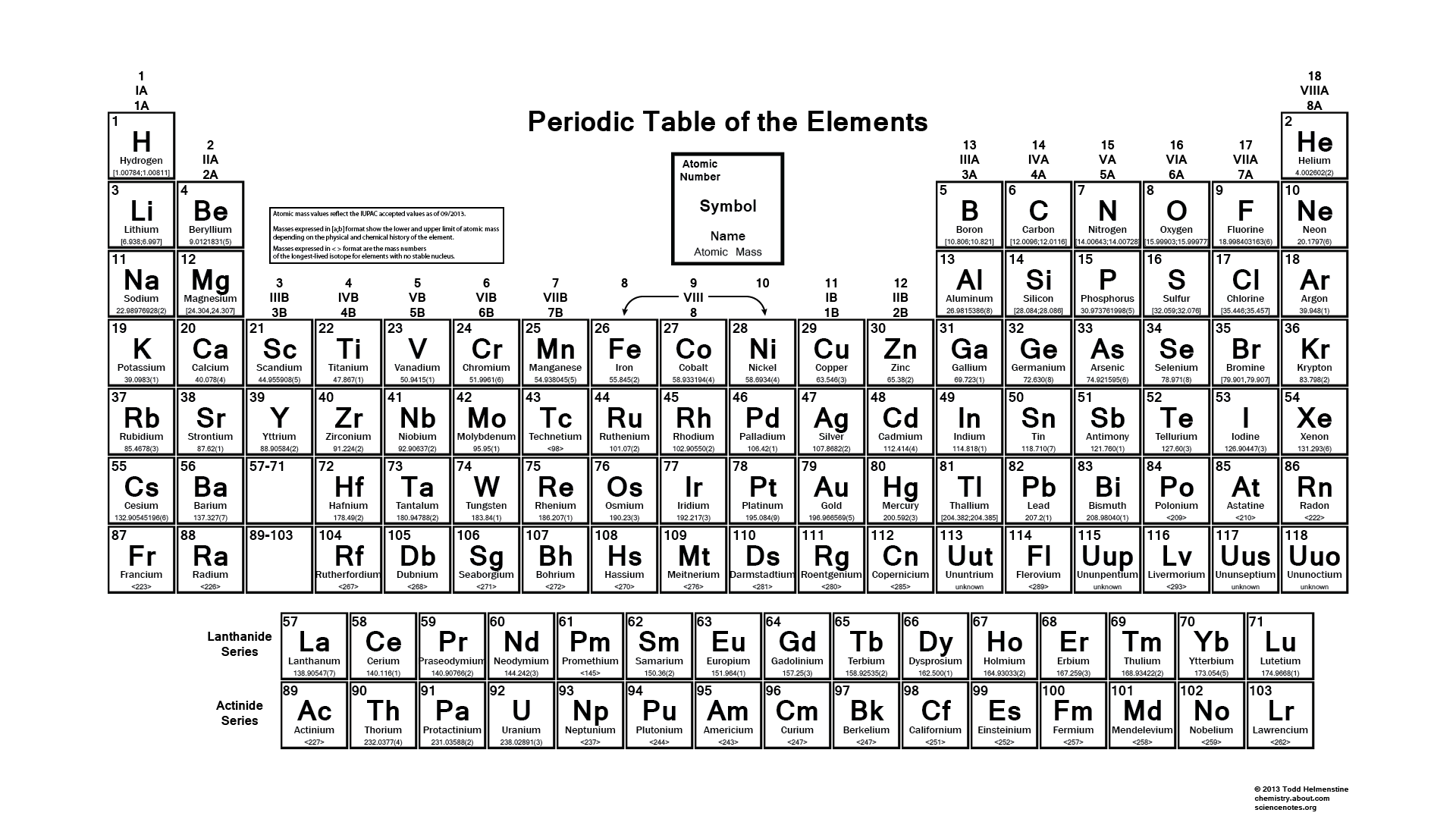
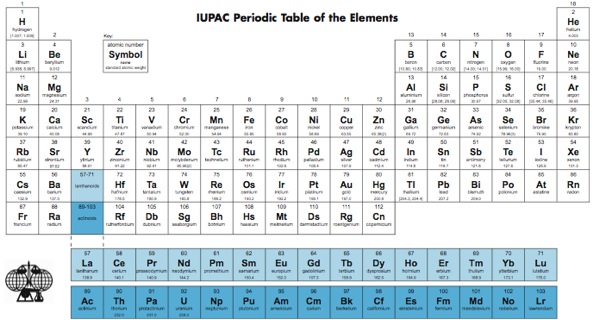
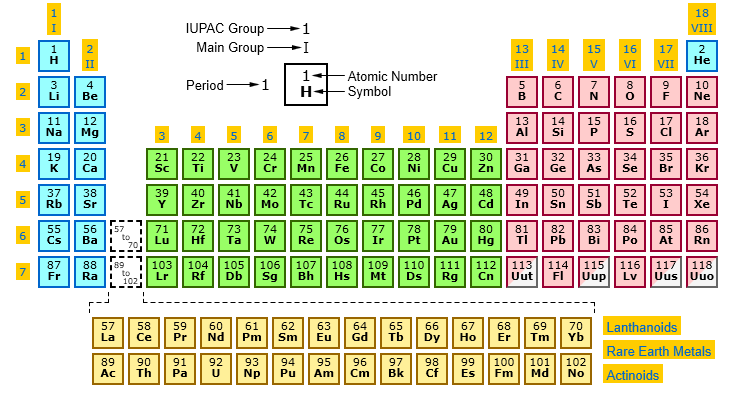
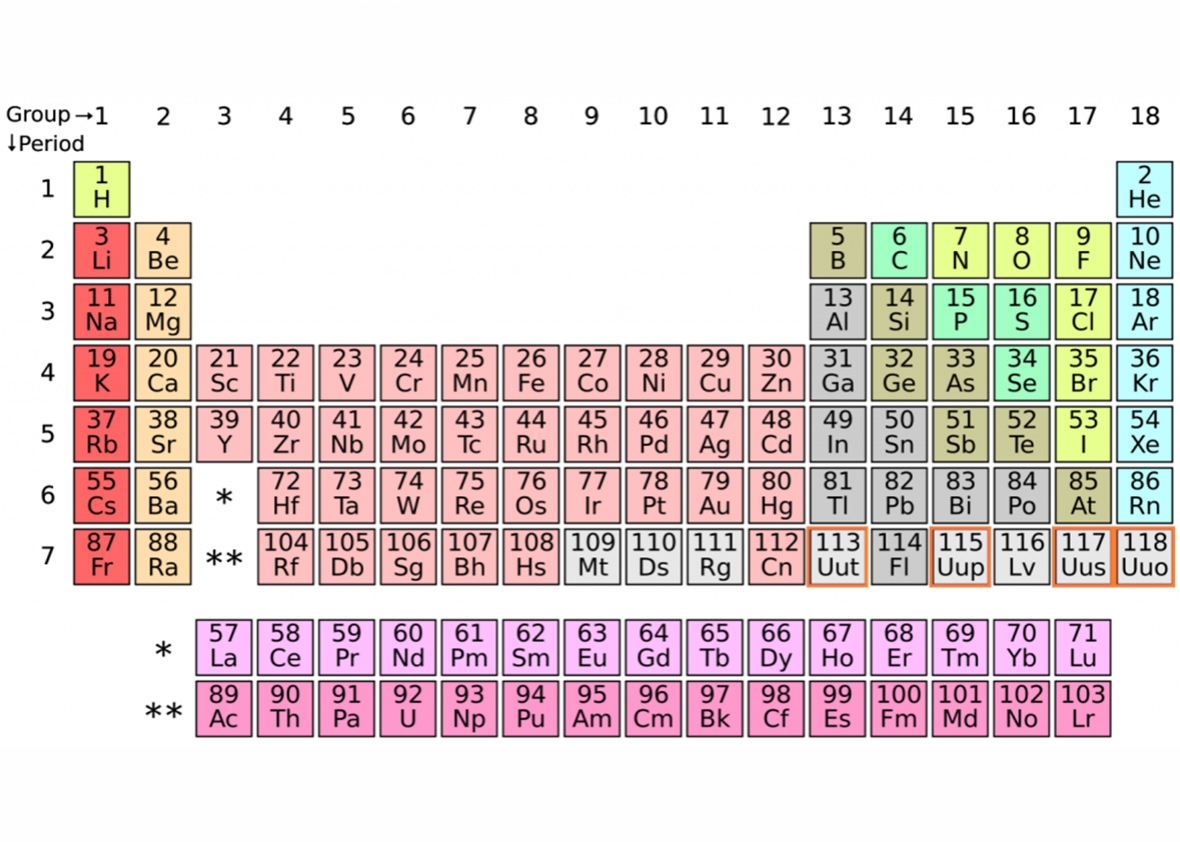
Elements With Names and Symbols – In the periodic table of elements, 118 chemical elements are arranged in a table format according to scientists ordered by atomic number, electrons, and also with names and symbols. The periodic Table is prepared in such a way that on the right side Nonmetals are arranged and on the left side, Metals are arranged.
All the elements in the table from 1-118 were either discovered or synthesized. The discovery of these 118 chemical elements was made in mid 19th century.
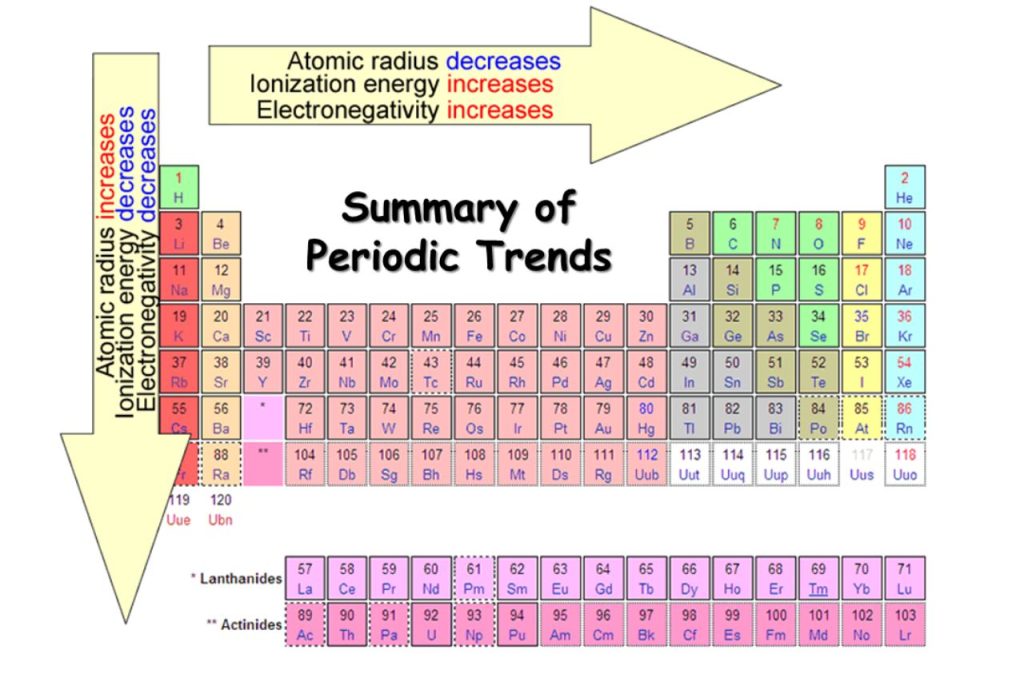
Elements With Names and Symbols
The elements found in the periodic table are the fundamental building blocks of matter. Each element is identified by a unique name and symbol, making it easier for scientists to communicate and reference these elements in various contexts. Elements are organized based on their atomic number, which represents the number of protons in the nucleus of an atom. The periodic table is divided into periods (rows) and groups (columns), allowing for classification based on similar properties and behaviors.
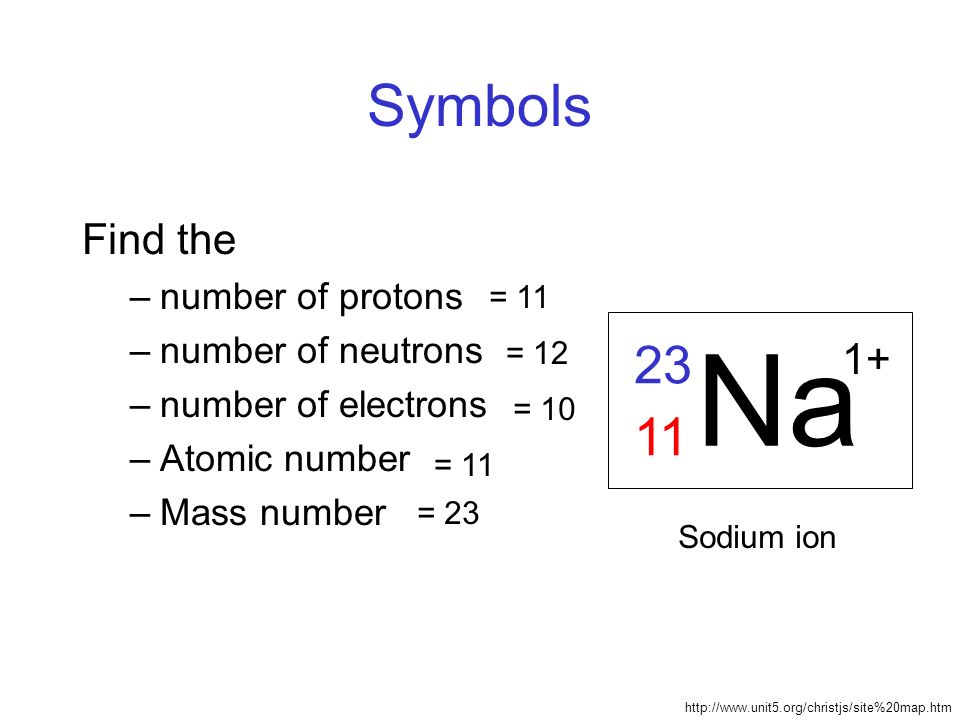
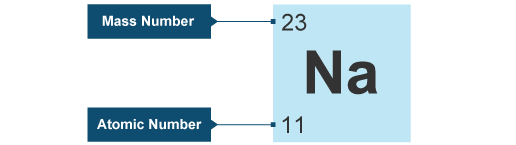
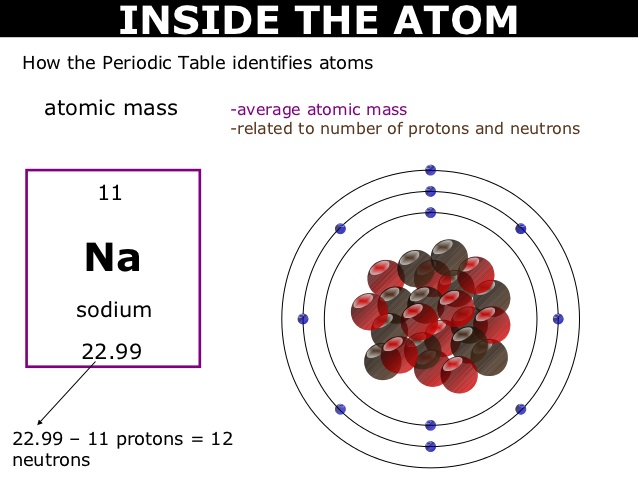
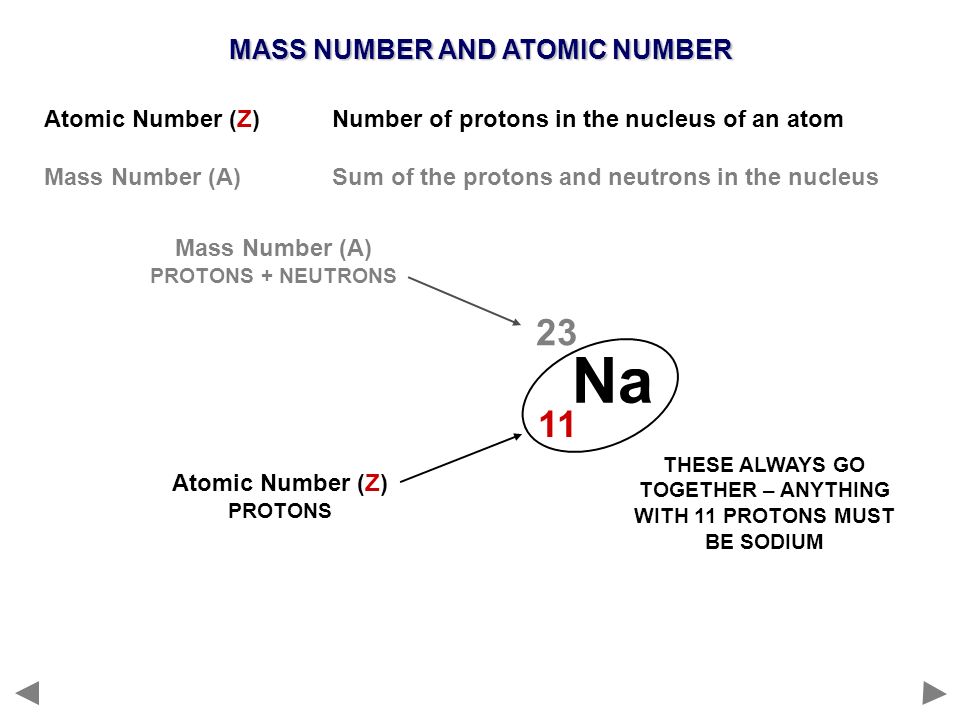
For example, hydrogen, the lightest element, has the symbol ‘H’ and atomic number 1. Oxygen, a crucial element for life, is represented by ‘O’ and has atomic number 8. The element carbon, which forms the backbone of organic compounds, is denoted as ‘C’ with an atomic number of 6. These symbols are not arbitrary; derived from the element’s name, often in Latin or Greek, to provide a convenient shorthand for scientists across the world. Learn What is Sodium Mass Number and Atomic Number here.
Transition metals like iron (‘Fe’) and copper (‘Cu’) have symbols derived from their Latin names, “Ferrum” and “cuprum,” respectively. These names hark back to the historical roots of chemistry. Furthermore, noble gases like helium (‘He’) and neon (‘Ne’) boast symbols that reflect their inert nature. Elements like carbon (‘C’) and oxygen (‘O’) have straightforward symbols that direct representations of their names. This combination of historical, linguistic, and practical elements in symbols makes the periodic table a masterpiece of scientific communication.
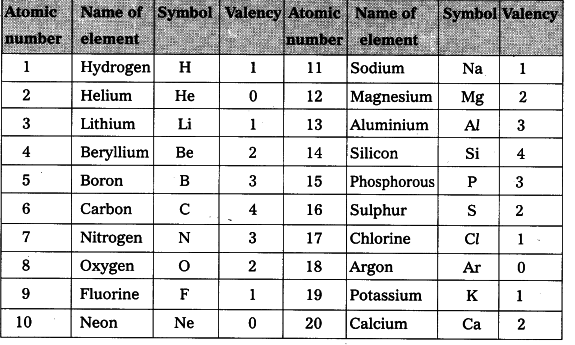
What are the 20 elements and their Symbols?
The first 20 elements in the periodic table offer a diverse array of properties and functions. Hydrogen (‘H’), the lightest element, fuels stars and forms the basis of water. Helium (‘He’) is known for its use in balloons and as a cooling agent in scientific experiments. Lithium (‘Li’) is utilized in rechargeable batteries due to its lightweight and high energy density. Beryllium (‘Be’) is valued for its strength-to-weight ratio, making it essential in aerospace applications.
Boron (‘B’) is a vital component in borosilicate glass and various compounds used in electronics. Carbon (‘C’) is unparalleled in its ability to form diverse molecules, from diamond to graphite to organic compounds. Nitrogen (‘N’) is crucial for life and is a main component of Earth’s atmosphere. Oxygen (‘O’) is essential for respiration and combustion, while fluorine (‘F’) is the most reactive of all elements.
Neon (‘Ne’) is commonly used in neon lights due to its distinctive orange-red glow. Sodium (‘Na’) and chlorine (‘Cl’) combine to form table salt, a staple in our diets. Magnesium (‘Mg’) is abundant in Earth’s crust and plays a vital role in biological processes. Aluminum (‘Al’) is lightweight and corrosion-resistant, finding use in everything from aircraft to beverage cans. Silicon (‘Si’) is the basis of modern electronics, while phosphorus (‘P’) is a key element in DNA and cell membranes.
Sulfur (‘S’) is known for its distinctive smell and is used in various industrial processes. Chlorine (‘Cl’) is a powerful disinfectant and essential for water purification. Potassium (‘K’) is crucial for nerve and muscle function, while calcium (‘Ca’) is integral for bone health. Scandium (‘Sc’) is used in some aerospace applications due to its lightweight and strength.
Periodic Table Symbols
Periodic table symbols shorthand notations that represent elements. Derived from the element’s name, these symbols play a pivotal role in chemistry and science communication. The first letter is capitalized, and if a second letter is present, it’s lowercase. For instance, ‘O’ stands for oxygen, and ‘N’ represents nitrogen. Elements like iron (‘Fe’) and copper (‘Cu’) have symbols taken from their Latin names.
These symbols simplify the representation of chemical compounds and reactions. They enable scientists to express complex concepts succinctly and facilitate international collaboration. Symbols have historical and linguistic significance, bridging gaps between languages and ensuring uniformity in scientific discourse.
Sodium (‘Na’) is derived from the Latin word “atrium,” and potassium (‘K’) is derived from “kalium.” Copper’s (‘Cu’) symbol is derived from “cuprum,” while silver’s (‘Ag’) symbol comes from “argentum.” Iron (‘Fe’) originates from “ferrum,” and lead (‘Pb’) traces its symbol back to “plumbum.” The periodic table’s symbols are a testament to the interconnectedness of language, history, and science.
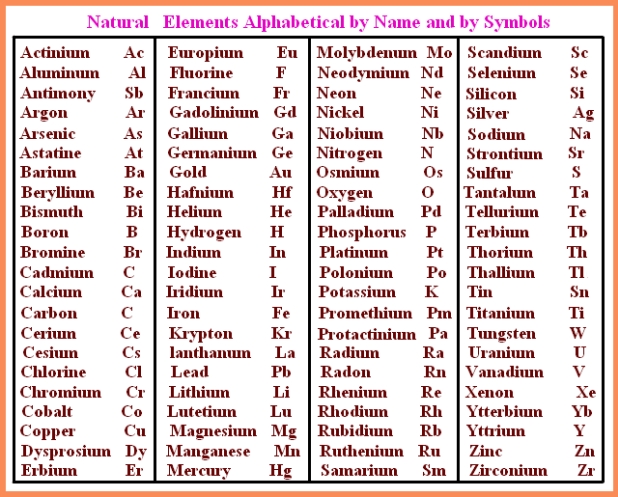
Periodic Table Full Names and Symbols
The periodic table’s rich tapestry extends beyond symbols; each symbol often carries a story that connects us to history and discovery. Silver, symbolized by ‘Ag,’ comes from the Latin “argentum,” reflecting its use since ancient times in jewelry and currency due to its lustrous appearance. The symbol ‘Au’ for gold is derived from the Latin “aurum,” honoring its timeless allure.
Elements like nitrogen (‘N’) and oxygen (‘O’) have straightforward symbols that mirror their English names, maintaining simplicity for ease of use. Some symbols, such as ‘Fe’ for iron and ‘Cu’ for copper, date back to ancient times when Latin was the universal language of scholars. The symbol ‘Hg’ for mercury finds its roots in the Roman god Mercury, known for speed and mobility, much like the liquid metal.
As scientists uncovered and synthesized new elements, the tradition of symbolic naming continued. Elements like Americium (‘Am’) pay homage to the United States, while Californium (‘Cf’) commemorates the state of California and the Lawrence Berkeley National Laboratory. This fusion of scientific discovery and historical context creates a captivating narrative within the periodic table.