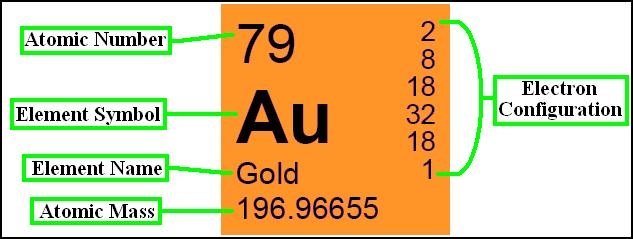
Hello Friends! In this article, you will get the detailed knowledge of Atomic Number and Mass Number. The atomic number is the total numbers of the of protons in the nucleus present of an atom which gives you atom number and is represented by capital Z. The combination of neutrons and protons that gives you a mass number of an atom, a combination of both are called nucleons.
Atomic Number and Mass Number
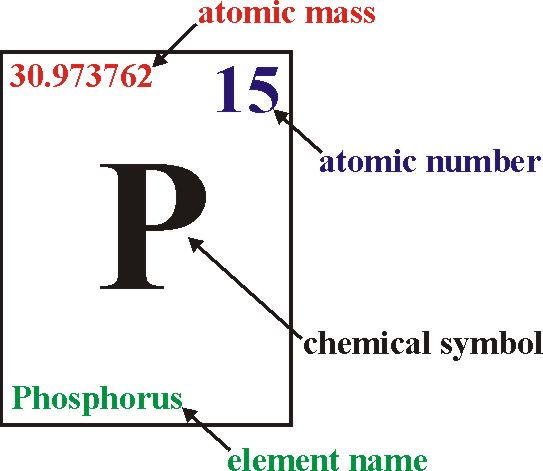
The atomic number of an element is a fundamental concept in chemistry, representing the number of protons found in the nucleus of an atom of that element. It defines the unique identity of an element since each element has a distinct number of protons in its nucleus.
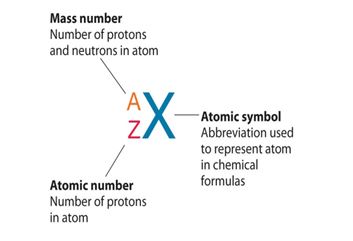
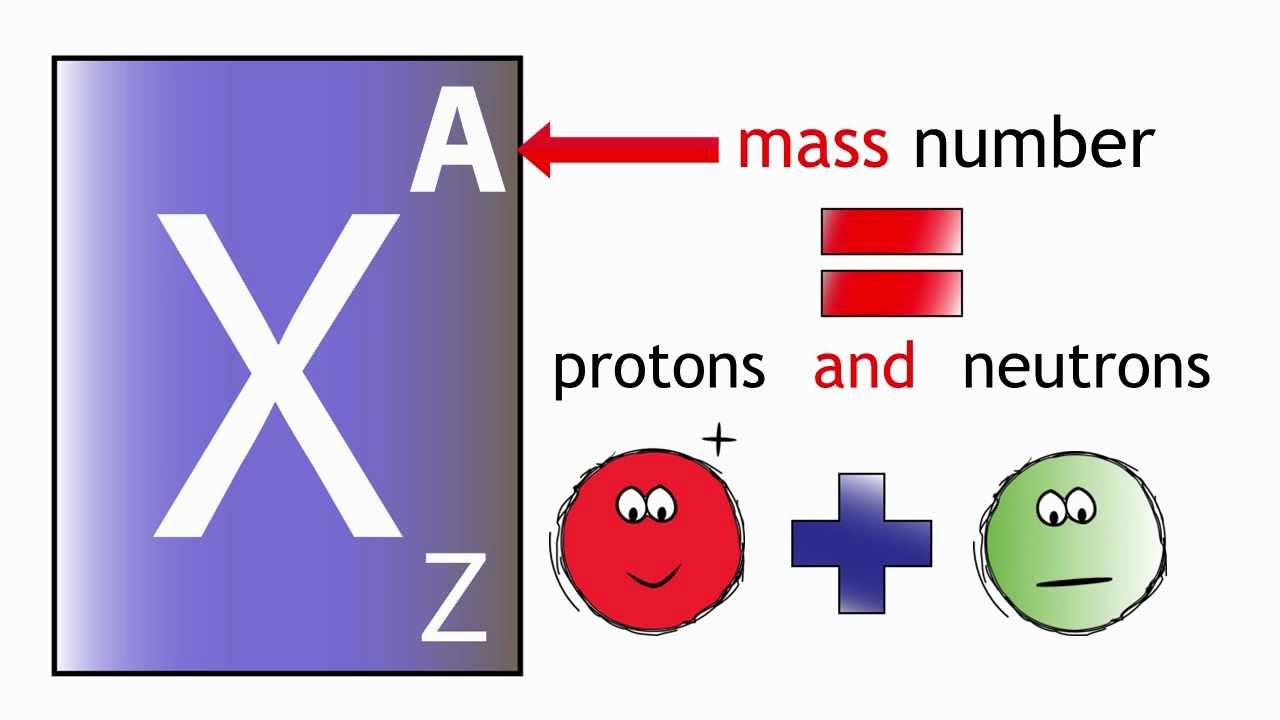
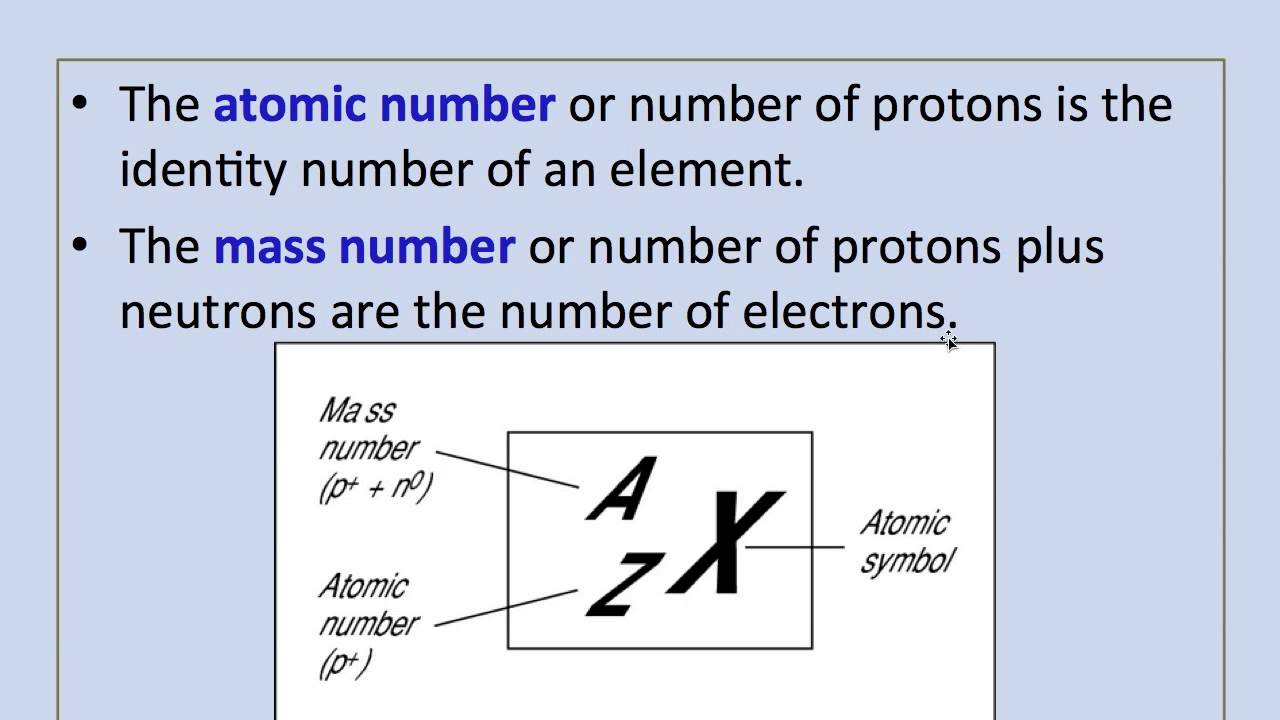
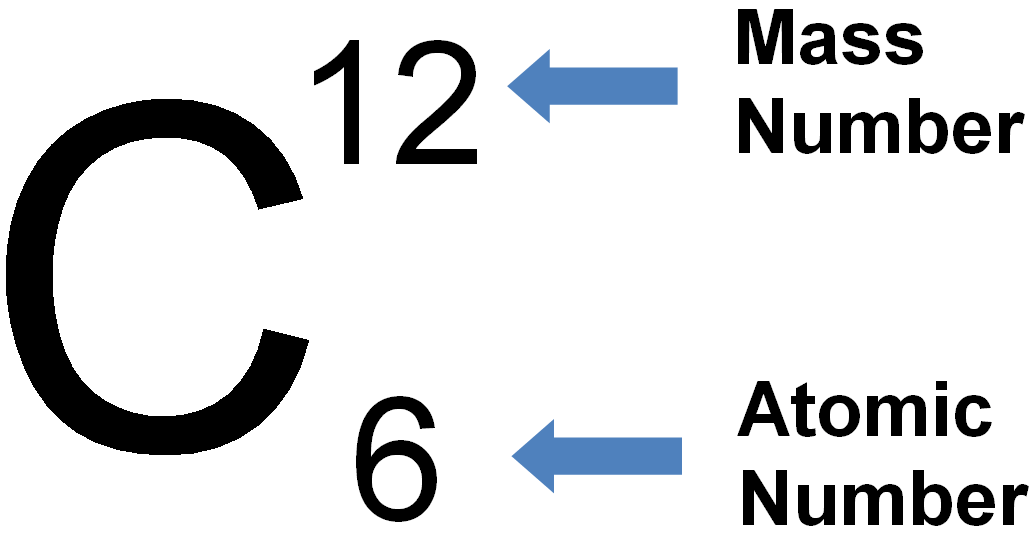
Mass Number: The mass number, denoted by the symbol “A,” represents the total number of protons and neutrons in the nucleus of an atom of a specific element. Since neutrons and protons have almost equal mass, the mass number is approximately equal to the total number of nucleons (protons and neutrons) in an atom. While the atomic number defines the element itself, the mass number may vary among atoms of the same element due to the existence of isotopes—atoms with the same atomic number but different numbers of neutrons. Isotopes can have different physical properties but share similar chemical behavior.
Relationship between Atomic Number and Mass Number: The relationship between atomic number (Z) and mass number (A) is simple. The atomic number (Z) only represents the number of protons, whereas the mass number (A) takes into account both protons and neutrons. Thus, to find the number of neutrons in an atom of an element, you can simply subtract the atomic number from the mass number: Number of Neutrons (N) = Mass Number (A) – Atomic Number (Z).
Importance of Atomic Number and Mass Number: The atomic number is crucial in distinguishing one element from another and is the basis for organizing the periodic table. On the other hand, the mass number helps identify isotopes and plays a significant role in nuclear chemistry and reactions. Understanding both atomic and mass numbers is essential for comprehending the behavior of elements in various chemical and physical processes.
Atomic Number Periodic Table
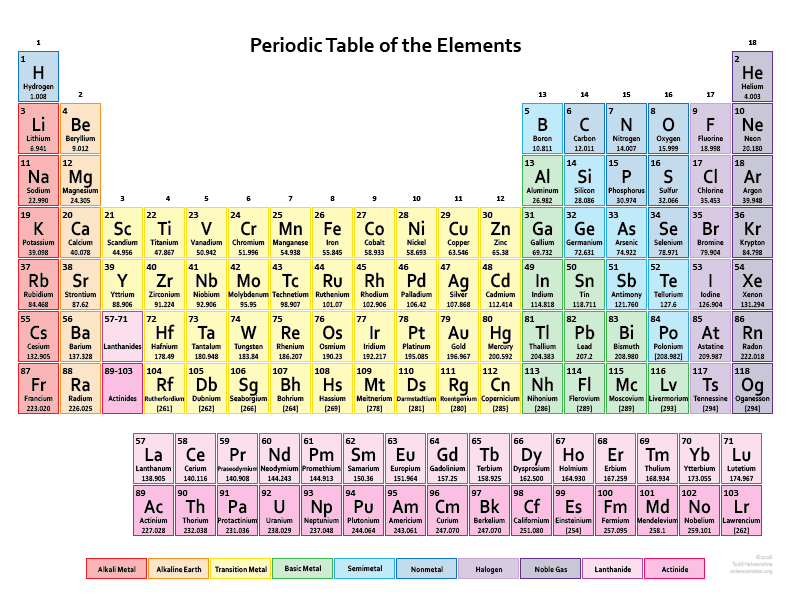
The periodic table is a comprehensive tabular arrangement of all known chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. The atomic number plays a vital role in the layout of the periodic table. The elements are placed in ascending order of atomic number, from left to right and top to bottom. This arrangement results in the elements with similar chemical behavior falling into the same vertical columns, known as groups or families.
The periodic table comprises several periods (horizontal rows) and groups (vertical columns). Each period represents the energy levels or shells occupied by electrons in an element’s atom, and each group shares similar electron configurations and chemical properties. Elements in the same group tend to have the same number of valence electrons, which contributes to their similar chemical reactivity.
In addition to organizing elements based on atomic number, the periodic table provides crucial information about each element’s name, symbol, atomic mass, and electron configuration. This information is valuable for scientists and students alike, as it allows for a systematic understanding of the properties and behavior of elements.
The discovery and development of the periodic table have been one of the most significant achievements in the history of chemistry. It provides a concise and insightful representation of the vast array of chemical elements found in the universe, enabling scientists to predict and understand the properties of elements that have not yet been synthesized or discovered. Learn What are the Understanding of Electron Cloud Model here.
How To Find Atomic Number
Finding the atomic number of an element is a straightforward process, and it can be done through several methods. The atomic number is typically listed for each element on the periodic table, making it readily available for reference.
However, if you encounter an element without the atomic number specified, there are several ways to find it:
- Periodic Table: As mentioned earlier, the atomic number of an element can be directly obtained from the periodic table. Look for the element’s symbol on the table, and the atomic number is the whole number located above or below the symbol.
- Proton Count: Since the atomic number represents the number of protons in the nucleus of an atom, you can determine it by counting the protons in the atom. Each proton carries a positive charge, and the atomic number specifies the number of these positive charges within the nucleus.
- Electron Configuration: Alternatively, you can use the electron configuration of the element to find its atomic number. The electron configuration is a shorthand notation representing the distribution of electrons in the atom’s energy levels. The atomic number is equal to the number of electrons in a neutral atom of the element.
- Mass Number and Charge: In case you have the mass number (total number of protons and neutrons) and charge of the atom, you can calculate the atomic number using the equation: Atomic Number (Z) = Mass Number (A) – Charge.
Remember that each element has a unique atomic number, which defines its identity and position in the periodic table. The atomic number is a fundamental property of an element and serves as a crucial factor in determining its chemical behavior.
Atomic Number Examples
Atomic number is a fundamental concept in chemistry, and understanding it is essential for working with elements and their properties.
Let’s take a look at some examples of atomic numbers for various elements:
- Hydrogen (H) – Atomic Number: 1: Hydrogen, the lightest and most abundant element in the universe, has an atomic number of 1. This means that a hydrogen atom contains only one proton in its nucleus.
- Carbon (C) – Atomic Number: 6: Carbon, a non-metallic element found in all living organisms, has an atomic number of 6. It contains six protons in its nucleus.
- Oxygen (O) – Atomic Number: 8: Oxygen, a vital element for supporting life, has an atomic number of 8. It contains eight protons in its nucleus.
- Iron (Fe) – Atomic Number: 26: Iron, a transition metal with various industrial applications, has an atomic number of 26. It contains 26 protons in its nucleus.
- Uranium (U) – Atomic Number: 92: Uranium, a radioactive element used in nuclear reactors and weapons, has an atomic number of 92. It contains 92 protons in its nucleus.
These examples illustrate the diversity of atomic numbers for different elements. Remember that the atomic number is the fundamental property that defines an element and determines its unique position in the periodic table.
What Is The Atomic Number Of An Element?
The atomic number of an element is a fundamental property that defines the element and distinguishes it from all other elements. It represents the number of protons found in the nucleus of an atom of that particular element. Each element on the periodic table has a distinct atomic number, which is a whole number and is denoted by the symbol “Z.”
The atomic number plays a crucial role in determining an element’s chemical properties and its position in the periodic table. Elements with the same atomic number belong to the same chemical element, irrespective of their atomic mass or number of neutrons. For example, all atoms with six protons in their nuclei are carbon atoms, regardless of whether they have six, seven, or eight neutrons, resulting in different isotopes of carbon.
The atomic number provides valuable information about the element’s electronic configuration and the number of electrons in its atom. For neutral atoms, the atomic number is equal to the number of electrons surrounding the nucleus. This property influences an element’s reactivity, as it determines the element’s ability to gain, lose, or share electrons during chemical reactions.
In summary, the atomic number is a fundamental concept in chemistry that defines an element, its position in the periodic table, and its chemical properties. It serves as a vital tool for scientists and chemists in understanding the behavior of elements and their interactions in various chemical processes.
What Is The Definition Of Atomic Number?
The atomic number is a fundamental concept in chemistry, representing the number of protons found in the nucleus of an atom of a specific chemical element. It is denoted by the symbol “Z” and is a whole number that distinguishes one element from another. In a neutral atom, the atomic number is also equal to the number of electrons surrounding the nucleus.
The concept of atomic number was first proposed by Henry Moseley, a British physicist, in 1913. He realized that organizing elements based on their atomic masses (as done previously) did not produce a clear pattern of chemical properties. However, arranging elements by their atomic numbers led to a more systematic and periodic pattern, laying the foundation for the modern periodic table.
The atomic number serves as the unique identifier for an element and plays a vital role in understanding its chemical behavior. And then the elements with the same atomic number share similar chemical properties, as they have the same number of valence electrons, which determines their reactivity. Moreover, the atomic number defines an element’s position in the periodic table, as elements arranged in ascending order of atomic numbers, resulting in periods and groups with similar properties.
In summary, the atomic number is a crucial concept in chemistry that defines an element’s identity, its position in the periodic table, and its chemical behavior. It serves as the foundation for the systematic classification and study of the vast array of elements present in the universe.
What Is An Atomic Number In Chemistry?
In chemistry, the atomic number is a fundamental concept that defines the unique identity of a chemical element. It represents the number of protons found in the nucleus of an atom of that particular element. This concept introduced to bring a more systematic order to the elements and their properties.
The atomic number plays a crucial role in the periodic table, which is a tabular arrangement of all known elements based on their atomic number, electron configuration, and chemical properties. Elements with the same atomic number placed in the same vertical column, known as a group, which means they exhibit similar chemical behavior. For example, all elements with an atomic number of 8 belong to Group 16 and referred to as the oxygen family.
The atomic number is a whole number, and it is unique to each element. For instance, hydrogen, the lightest element, has an atomic number of 1, indicating it contains only one proton in its nucleus. In contrast, elements with higher atomic numbers, such as uranium with an atomic number of 92, have more protons in their nuclei.
The atomic number is also essential for understanding isotopes. Isotopes are atoms of the same element that have the same atomic number but different mass numbers due to varying numbers of neutrons in the nucleus. The atomic number allows scientists to differentiate between isotopes of an element and study their various physical and chemical properties.
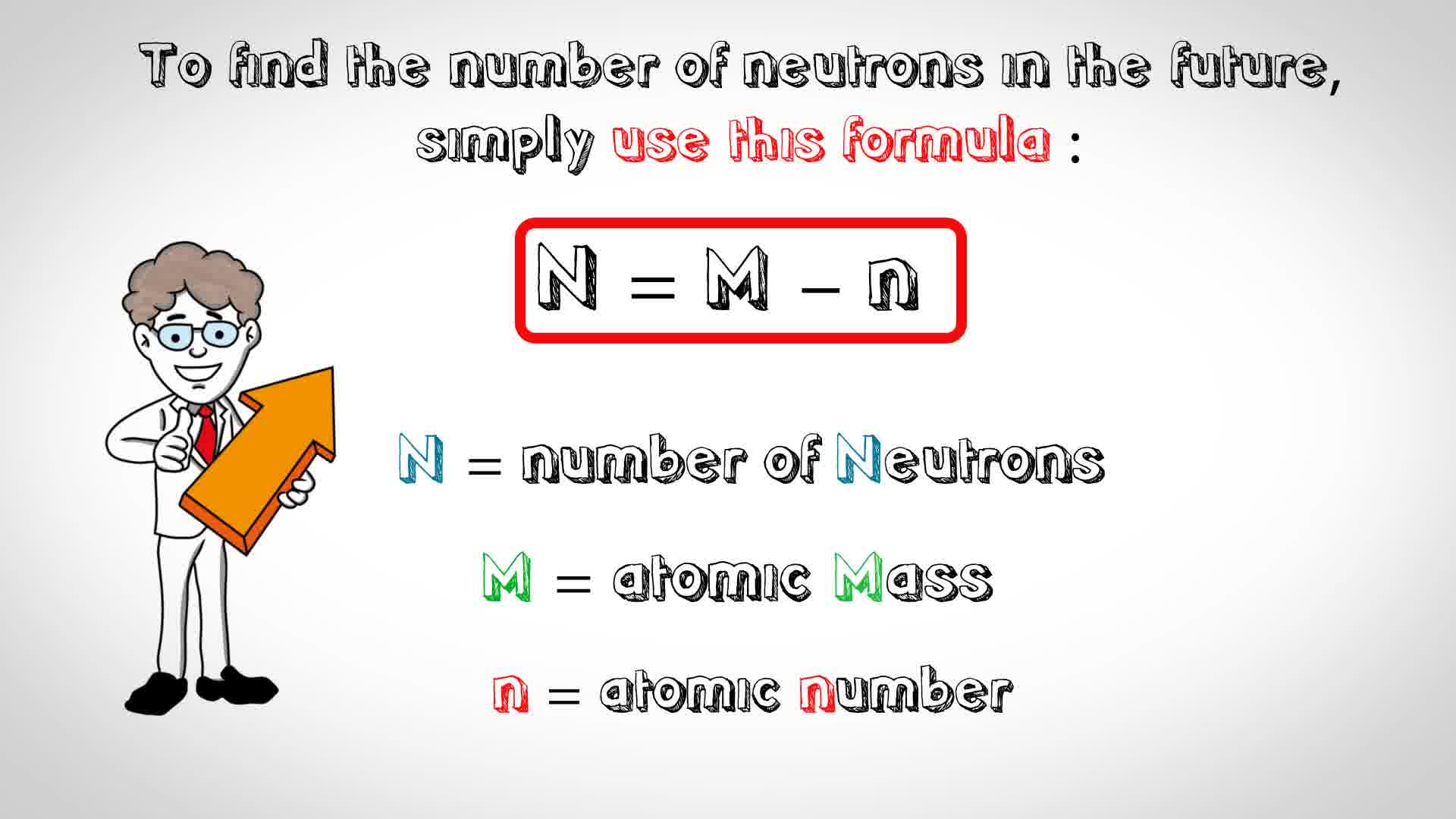
Leave a Reply