Study the Radium electron configuration here in the article and build a solid understanding of the element for your chemistry class. Here in the article, we shall provide the electron configuration and the other important properties of this element.
Radium is one of the most commonly known chemical elements in chemistry. It has the atomic number 88 and the symbolic sign of Ra. The element belongs to the group 2 category of the periodic table as the sixth element. The element also owns its root from the category of Alkaline earth metals.
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
Radium has a physical structure as a white and silvery object and can easily react with nitrogen. The chemical element is highly radioactive in nature and even all of the isotopes of the element are also radioactive. Radium generally has a very long life which is about 1600 years. In its decayed state, the element leaves behind the ionizing radiation as its by-product.
Radium was first discovered in the year of 1898 by the two scientists together during the mining process. In modern times, the majority of the Radium comes from the ores of Uranium and Thorium. The chemical element is not at all required for any of the living organisms including humans. The accident or the sudden consumption of the element can cause some serious health effects.
Radium Electron Configuration
The Radium electron configuration is a significant part of the study of the element. In fact, without understanding the electron configuration of Radium the element remains unknown. So, basically, the electron configuration of Radium is the process in which Radium distributes its electrons to the atomic orbitals of the element. This is the natural tendency of all types of chemical elements and they all have their respective electron configuration.
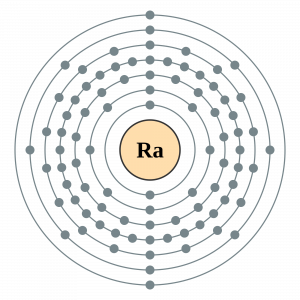
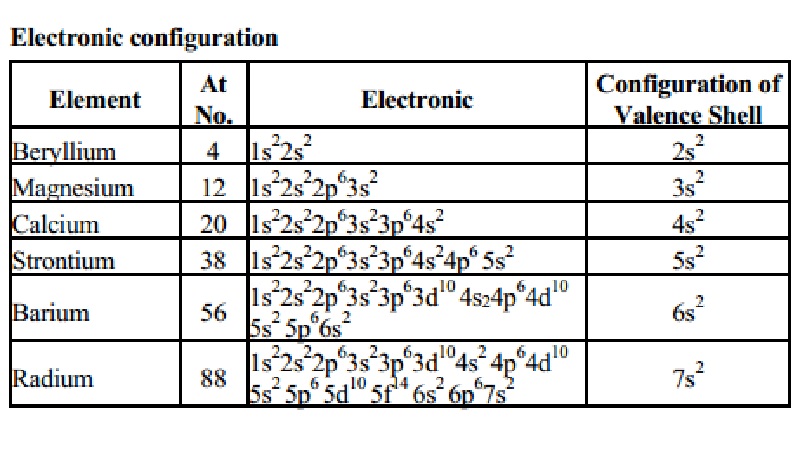
So, the electron configuration of Radium is Rn 7s2 in its short abbreviated form after applying the formula for the same.
The process of the electron configuration further helps in the overall breakdown of the element. It subsequently helps in understanding the chemical element in a thorough manner.
Here below are some of the major usages of the electron configuration of Radium.
- Electron configuration helps in determining the valency of Radium.
- We can also determine the reaction properties of Ra with the help of its electron configuration.
- The chemical bonding of Radium also heavily depends upon the electron configuration of element.
How many valence electrons does Radium have?
Well, as we have discussed that Radium is basically a chemical element that has the majority of radioactive chemical properties. Being one of the oldest chemical elements it’s quite familiar to mankind around the world. For the same reason, the majority of the usage of Radium is already known. The major usage of Radium in the present time goes to atomic, and molecular energy.
Electron Configuration for Ra
It’s also useful in the domain of optical physics. Furthermore, with its radioactive properties, the element is also useful as the source of radiation in several radiography industries. It’s one of the most promising and easily available chemical elements in chemistry. We hope that the article would provide some useful awareness of the element to readers.
Leave a Reply