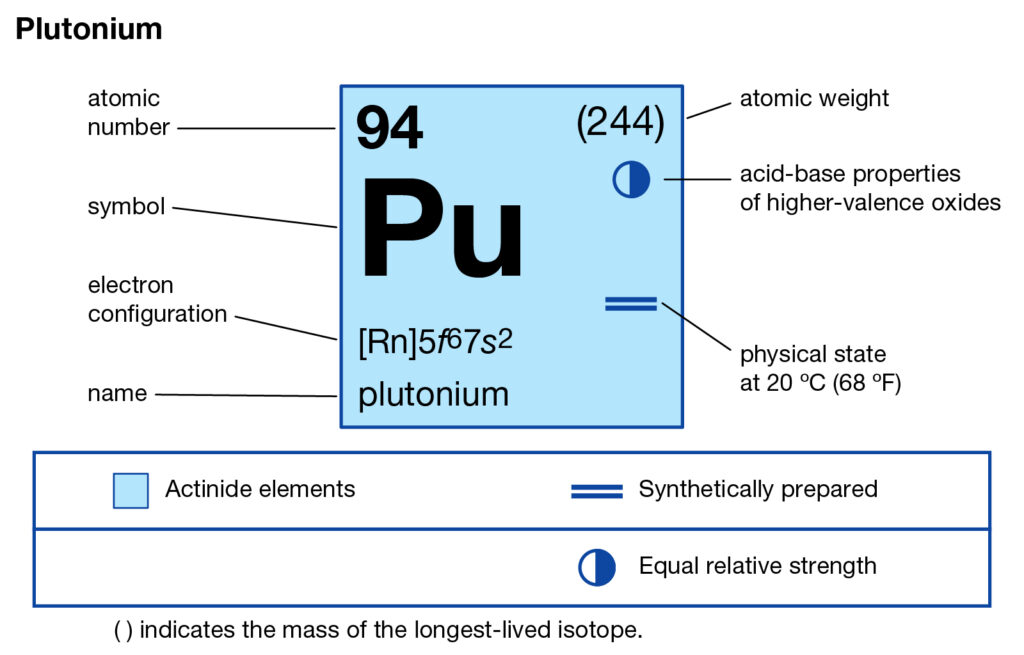
We are providing the study of Plutonium valence electrons here in the article. You can read and explore the element with better insight in the article. In chemistry, plutonium is one of the highly radioactive chemical elements. It has the atomic number 94 and the representative symbol as Pu.
How many valence electrons does Plutonium have?
It has the physical appearance of the actinide silver grayish metal. Plutonium has to tarnish properties in the exposure of air. Plutonium further holds high reaction properties as well. It hence easily reacts with hydrogen, nitrogen, silicon, carbon, etc gases. Plutonium being the synthetic chemical element has very little form in nature. It was first discovered in the year 1940 by bombarding Uranium.
Some form of Plutonium is available in the naturally occurring Uranium. It is hence the only natural chemical element that has the highest atomic. Being a highly radioactive chemical element Plutonium is highly dangerous to deal with. It is advisable to use only in the proper laboratory by professional scientists.
So, the major usage of plutonium includes explosives around the world. In fact, the world’s first nuclear bomb had plutonium cores in it. In the present scenario as well Plutonium is highly useful in the production of explosives. It is also useful in emitting thermal energy for various usages.
Plutonium Valence Electrons Dot Diagram
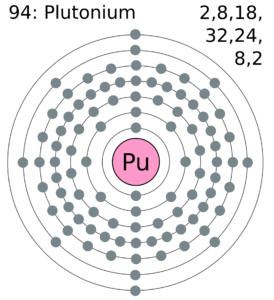
Readers can explore the Plutonium valence electrons with the help of a dot diagram. The diagram exclusively draws the numbers of dots around the symbol of Plutonium.
You can consider these dots as are the numbers of valence electrons. Moreover, you can explore whether there is single or double bonding of valence electrons of the atom.
Valency of Plutonium
The precise valency of Plutonium is 2 since it has 2 valence electrons. Valency is the combining capacity of Plutonium with other elements.