Electron Configuration For Iron: Chemical element Iron has atomic number 26 and its symbol is “Fe” which comes from the Latin word Ferrum. This metal lies in the first transition series and most common element on the earth which is present on the earth’s inner and outer core. It is a 4th most common element present on earth’s core.
Related Post –
- Sodium Electron Configuration
- Germanium Electron Configuration
- Nitrogen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
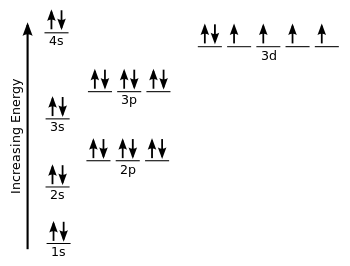
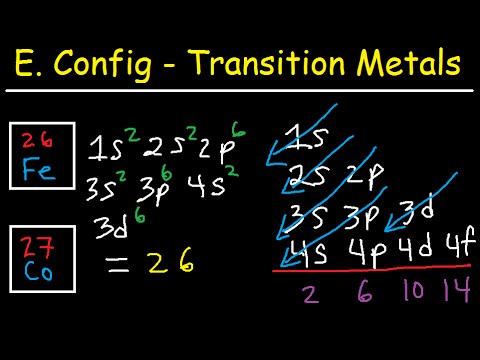
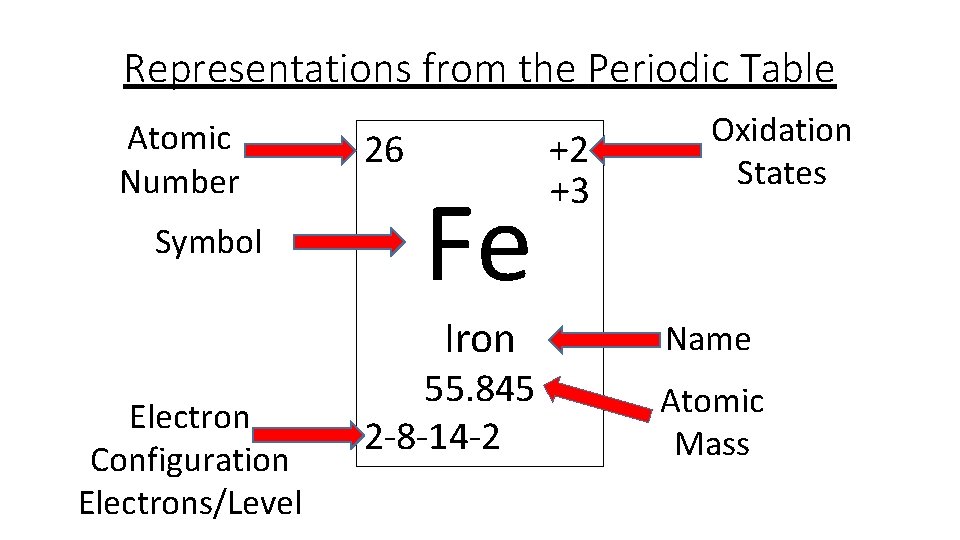
The electron configuration of iron can be defined as the distribution of electrons in orbit of atom or molecule. The electron configuration of iron has distribution of 26 electrons in 4 orbits of iron atom which can be defined as 1s22s22p63s23p63d64s2 or [Ar] 3d64s2.
Electron Configuration For Iron
-
Element Symbol: Fe
-
Atomic Number: 26
-
Full Configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s² -
Shorthand Configuration:
[Ar] 3d⁶ 4s² -
Valence Electrons: 2 (from 4s²)
-
Partially Filled Subshell: 3d⁶ (important for magnetism)
-
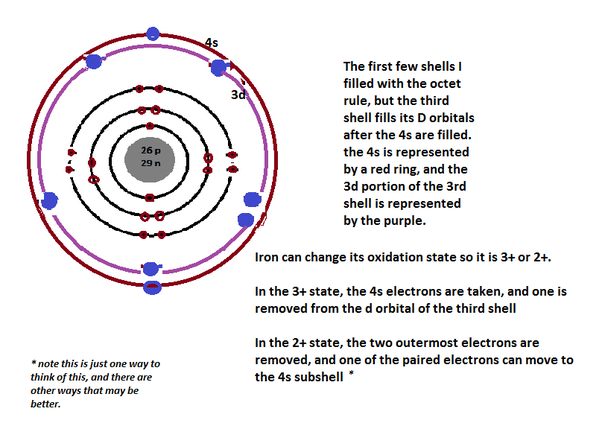
Common Oxidation States: +2, +3
Iron Number of Valence Electrons
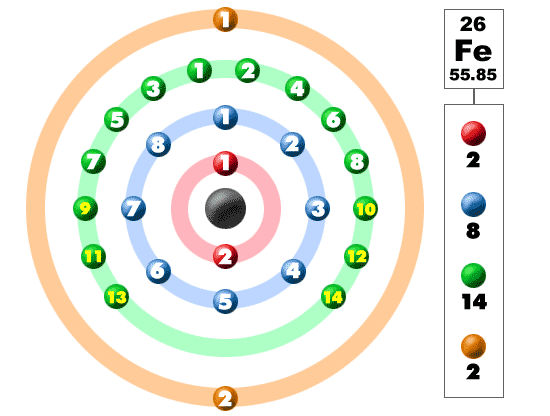
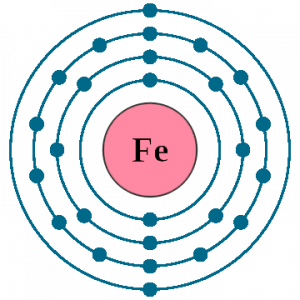
There are 2 electrons located in the outermost shell of iron which means iron has only 2 valence electrons.
Full Electron Configuration For Iron
There are 26 electrons of iron whose distribution of atom is 2, 8, 14, and 2 in its four orbits. And electron configuration can be represented as 1s22s22p63s23p63d64s2 or in terms of Argon it can also be represented as [Ar] 3d64s2.
Iron the most common element on earth has 26 electrons and e configuration of iron it can be defined as [Ar] 3d64s2 and it can also be defined as 1s22s22p63s23p63d64s2
How Many Valence Electrons Does Iron have
The number of valence electrons depends upon the number of electrons located in the outermost shell of an atom and in case of iron there are only 2 electrons present in the 4th or outermost shell. So, there are 2 valence electrons in iron.