Valency is a fundamental concept in chemistry that describes an element’s ability to combine with other elements and form compounds. The Valency For All the Elements determines the number of electrons it gains, loses, or shares when forming chemical bonds. This paragraph will provide an overview of the valency for all the elements in the periodic table, highlighting the diversity of chemical interactions and the significance of valency in predicting compound formation.
Valency For All the Elements
Valency is a fundamental concept in chemistry that describes the combining capacity or the number of bonds an element can form with other elements. It is crucial in understanding the formation of chemical compounds and predicting the behavior of elements in reactions. The valency of an element depends on its electron configuration and the number of valence electrons it possesses.
Valency varies for different elements across the periodic table. For example, elements in Group 1 of the periodic table, known as alkali metals, have a valency of +1. This means they readily lose one electron to achieve a stable octet configuration. Group 2 elements, the alkaline earth metals, have a valency of +2 as they tend to lose two electrons. Transition metals, found in the middle of the periodic table, can exhibit multiple valencies due to their ability to lose different numbers of electrons depending on the specific reaction.
Elements in Group 17, known as halogens, have a valency of -1. They readily gain one electron to achieve a stable octet configuration. Group 16 elements, the chalcogens, have a valency of -2, and Group 15 elements, the pnictogens, have a valency of -3. These elements tend to gain electrons to achieve a stable electron configuration.
In summary, the valency of elements varies across the periodic table based on their position and electron configuration. It is an essential concept for understanding chemical bonding and the formation of compounds. Learn more about Valency here:- Hassium Valence Electrons
Periodic Table with Valency
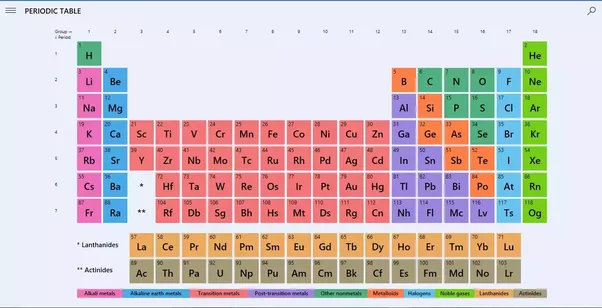
The periodic table of element arranges all the chemical elements in increasing order of their atomic number, recurring chemical properties and electron configuration. All the elements arranged in the tabular form. Metals placed on the left-hand side and non-metals placed on the right. Metallic property of the element decreases as one move from left to right.
The periodic table is a systematic arrangement of chemical elements based on their atomic number, electron configuration, and chemical properties. It provides valuable information about the valency of elements, which is crucial for understanding their behavior in chemical reactions and the formation of compounds.
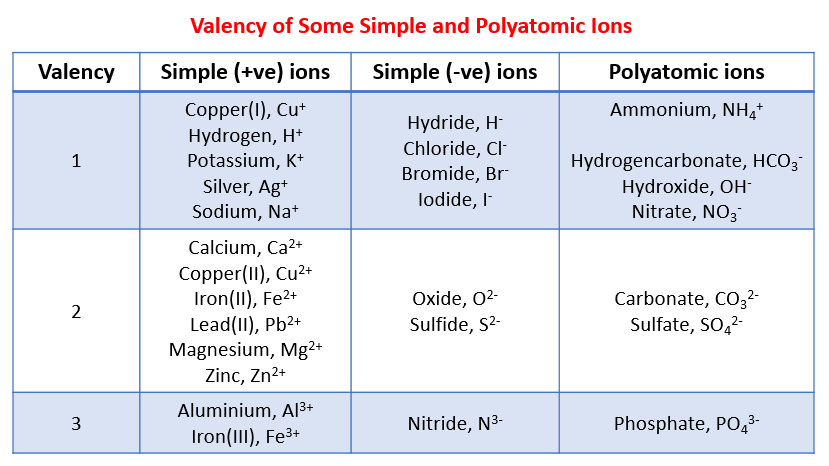
In a periodic table, the valency of elements is often indicated by a superscript or subscript number next to the element symbol. For example, Na^+ represents sodium with a valency of +1, while Cl^- represents chloride with a valency of -1. These symbols give a quick overview of the valency of elements and facilitate the prediction of their reactivity.
It’s important to note that while some elements have a fixed valency, others, especially transition metals, can exhibit multiple valencies. For such elements, Roman numerals used to indicate the specific valency. For example, Fe(II) represents iron with a valency of +2, and Fe(III) represents iron with a valency of +3.
By referring to the periodic table, scientists and students can easily identify the valency of different elements and use this information to predict their bonding behavior, formation of compounds, and participation in chemical reactions.
How to Find Valency of Elements
Determining the valency of elements is essential for understanding their chemical behavior and predicting their bonding patterns. The valency of an element is primarily determined by the number of valence electrons it possesses, which the electrons in the outermost energy level of an atom.
Here’s a general approach to finding the valency of elements:
- Identify the element’s position in the periodic table: The position of an element in the periodic table provides valuable information about its valency. Elements in Groups 1 and 2 typically have valencies of +1 and +2, respectively, while elements in Groups 16 and 17 often have valencies of -2 and -1, respectively.
- Determine the number of valence electrons: The valence electrons are typically represented by the electrons in the outermost energy level (shell) of an atom. The group number of an element generally represents the number of valence electrons it has, except for the transition metals. For example, elements in Group 1 have one valence electron, and those in Group 17 have seven valence electrons.
- Adjust the valency for transition metals: Transition metals can have variable valencies, so their valency is not solely determined by their group number. In such cases, additional information or the specific chemical compound is needed to determine the valency of a transition metal element.
- Consider the octet rule: The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight valence electrons. This rule applies to most elements, particularly non-metals. By understanding the octet rule, you can make predictions about the valency of element based on their tendency to gain or lose electrons.
By following these steps and referring to the periodic table, you can find the valency of most elements and gain insights into their chemical behavior and bonding patterns.
What is Valency with Examples
Valency is a fundamental concept in chemistry that describes the combining capacity or the number of bonds an element can form with other elements. It is determined by the number of valence electrons an element possesses. Valency plays a crucial role in chemical reactions and the formation of compounds.
Let’s explore valency through a few examples:
- Sodium (Na): Sodium is located in Group 1 of the periodic table, and it has one valence electron. To achieve a stable octet configuration, sodium readily loses this valence electron, resulting in a valency of +1. For example, when sodium reacts with chlorine (Cl), it donates its valence electron to form sodium chloride (NaCl).
- Oxygen (O): Oxygen is located in Group 16, which means it has six valence electrons. To achieve a stable octet configuration, oxygen tends to gain two electrons. Therefore, its valency is -2. In water (H2O), oxygen forms bonds with two hydrogen atoms by gaining two electrons from each hydrogen atom.
- Calcium (Ca): Calcium is an alkaline earth metal located in Group 2 of the periodic table. It has two valence electrons. To achieve a stable octet configuration, calcium tends to lose these two electrons, resulting in a valency of +2. For example, in calcium chloride (CaCl2), calcium donates its two valence electrons to two chlorine atoms.
- Carbon (C): Carbon is located in Group 14 and has four valence electrons. It can both gain and lose electrons to achieve a stable configuration. Carbon can form multiple compounds by sharing its electrons with other elements, resulting in various valencies depending on the specific compound.
These examples illustrate how valency determines the bonding behavior and reactivity of elements. By understanding the valency of different elements, scientists can predict the formation of compounds and how elements interact in chemical reactions.

Leave a Reply