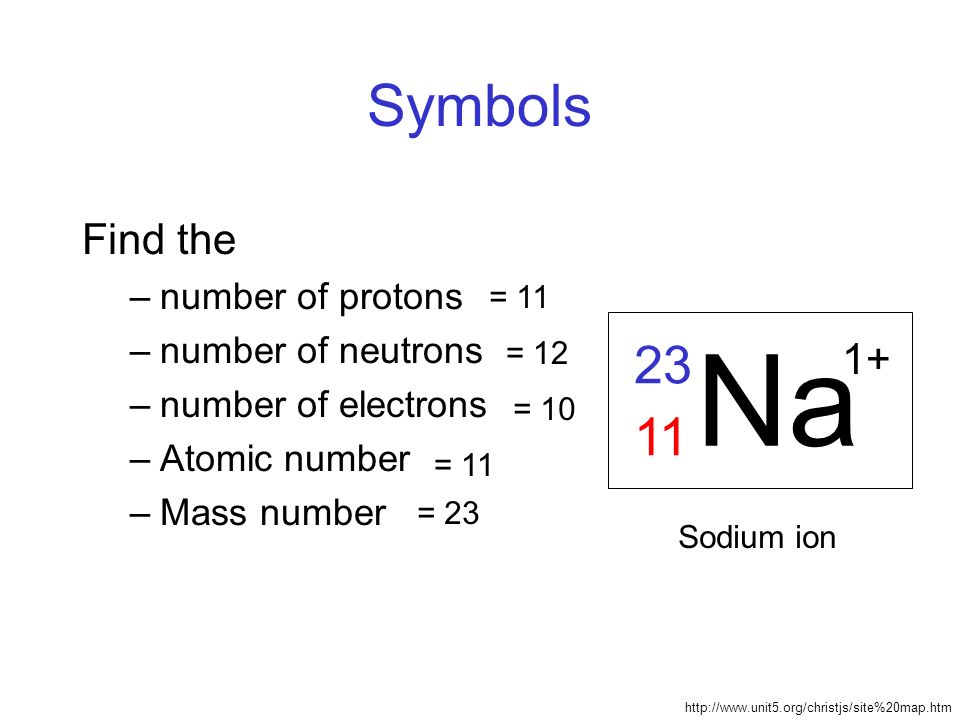
Sodium is a chemical element with its symbol NA and Sodium Mass Number and Atomic Number 11. It is silver-white, highly reacted and soft metal. Present in group 1 of the periodic table and it is alkali metal as it has a single electron on its outer shell.
It is most sixth abundant metal that exits on the earth’s crust. Many salts present in the sodium are highly soluble. It has its own importance for the plants and animals. Sodium is also used as a heat exchanger in some of the nuclear reactors. It has a low melting point. As its greyish white colour expose to air.
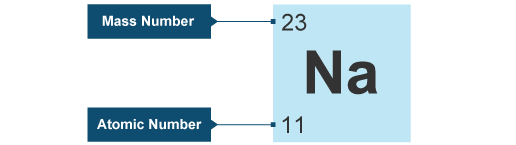
Sodium Mass Number and Atomic Number
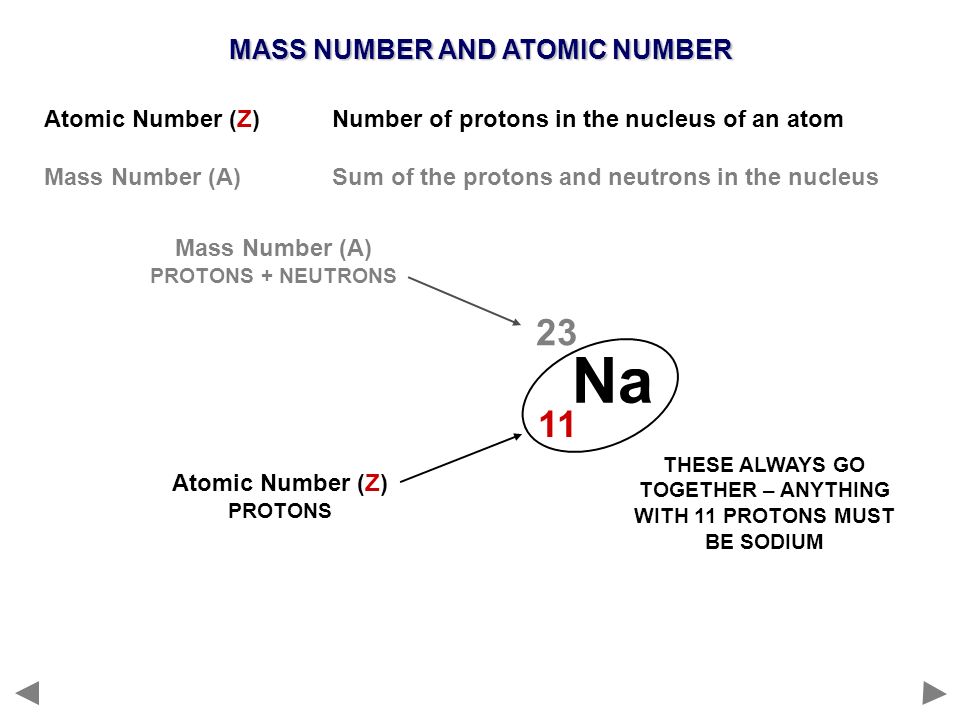
Sodium is a chemical element with the symbol Na and the atomic number 11. The atomic number of an element represents the number of protons found in the nucleus of an atom of that element. Since the number of protons determines an element’s identity, sodium’s atomic number of 11 signifies that every sodium atom contains 11 protons in its nucleus.
- Isotopes of Sodium: While sodium typically has an atomic number of 11, indicating 11 protons in its nucleus, it can have different mass numbers due to the presence of isotopes. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Some common isotopes of sodium include sodium-23, sodium-24, and sodium-22, with mass numbers 23, 24, and 22, respectively.
- Relative Abundance: The most abundant isotope of sodium found in nature is sodium-23, which makes up about 100% of naturally occurring sodium. Sodium-24, though less abundant, is also naturally occurring but in trace amounts. Other isotopes, such as sodium-22 and sodium-25, are produced artificially or found in minute quantities as products of nuclear reactions.
On the other hand, the mass number of sodium is not fixed and can vary based on the number of neutrons in its nucleus. The mass number is the sum of protons and neutrons in an atom’s nucleus. Given that sodium’s atomic number is 11 (indicating 11 protons), the number of neutrons in a sodium atom can be calculated by subtracting the atomic number from the mass number. Different isotopes of sodium can have varying numbers of neutrons, resulting in different mass numbers.
Sodium Number of Neutrons
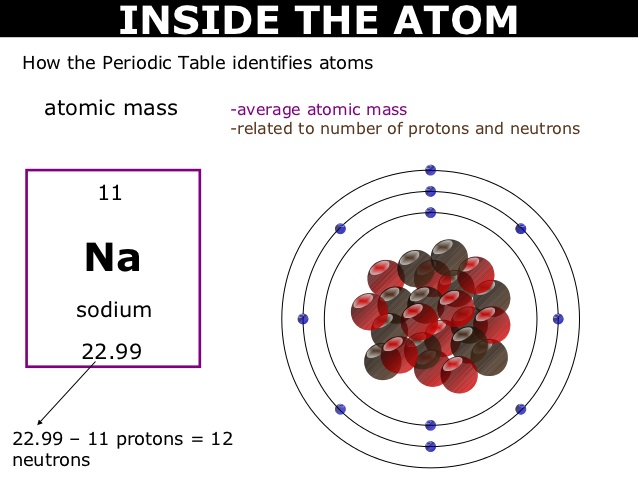
Sodium, with its atomic number of 11, typically has several isotopes with varying numbers of neutrons. To determine the number of neutrons in a sodium atom, we subtract the atomic number (which represents the number of protons) from the mass number (which represents the total number of protons and neutrons).
One of the most common isotopes of sodium is sodium-23. The mass number of sodium-23 is 23, and since sodium has an atomic number of 11, we can calculate the number of neutrons as follows:
Number of neutrons = Mass number – Atomic number Number of neutrons = 23 – 11 = 12
- Atomic Mass: The atomic mass of sodium is the weighted average of the masses of its naturally occurring isotopes, taking into account their relative abundances. For sodium, the atomic mass is approximately 22.99 atomic mass units (amu). The decimal fraction is due to the presence of various isotopes with slightly different masses.
- Isotopic Notation: Isotopes of sodium can be represented using isotopic notation, where the element’s symbol is followed by the sum of its atomic number (Z) as a subscript and its mass number (A) as a superscript. For example, sodium-23 is represented as ^23Na^11, where 23 is the mass number (A) and 11 is the atomic number (Z).
Therefore, sodium-23 has 12 neutrons in its nucleus. It is essential to note that other isotopes of sodium will have different numbers of neutrons.
Mass Number of a Sodium Atom
The mass number of a sodium atom is determined by adding the number of protons and neutrons present in its nucleus. As mentioned earlier, sodium has an atomic number of 11, representing the number of protons. The number of neutrons, on the other hand, can vary depending on the isotope of sodium.
Let’s consider the isotope sodium-24 as an example. Sodium-24 has an atomic number of 11, as all sodium isotopes do, and we can find its mass number by adding the number of protons and neutrons:
Mass number = Number of protons + Number of neutrons Mass number = 11 (protons) + 13 (neutrons) = 24
- Radioisotopes: Some isotopes of sodium, like sodium-24, are unstable and radioactive. These isotopes undergo radioactive decay, emitting radiation in the process. Radioisotopes of sodium find use in various applications, including medical imaging, radiotracer studies, and industrial processes.
- Neutrons and Stability: The number of neutrons in an atom’s nucleus plays a crucial role in its stability. Generally, stable isotopes have a balance of protons and neutrons in their nuclei. However, some isotopes may be unstable, leading to radioactive decay and the eventual transformation into more stable elements.
Therefore, the mass number of sodium-24 is 24. Different isotopes of sodium will have different mass numbers due to the varying number of neutrons they contain. Learn What Is Atomic Number And Mass Number? here.
What is a Possible Mass Number of a Sodium Atom
Sodium has several isotopes, each with a different number of neutrons, which results in various possible mass numbers for sodium atoms. The most abundant and stable isotope of sodium is sodium-23, which has 11 protons and 12 neutrons, giving it a mass number of 23. This isotope accounts for nearly 100% of naturally occurring sodium.
Apart from sodium-23, another possible mass number for a sodium atom is sodium-24. This isotope has 11 protons and 13 neutrons, resulting in a mass number of 24. Sodium-24 is less abundant compared to sodium-23 but is still found in trace amounts in nature.
- Formation in Stars: Sodium, like most elements heavier than helium, is primarily formed in stars through nuclear fusion. In the core of stars, hydrogen atoms fuse to form helium, releasing energy in the process. Further fusion reactions in more massive stars can produce elements like sodium, which are subsequently released into space through stellar processes like supernovae.
- Importance in Biology: Sodium is an essential element for biological organisms. It plays a critical role in maintaining proper fluid balance, nerve function, and muscle contraction. Sodium ions are crucial for the transmission of electrical signals in nerve cells and muscle cells, enabling essential bodily functions.
Other isotopes of sodium, such as sodium-22 and sodium-25, are possible as well, with different combinations of protons and neutrons leading to distinct mass numbers. These isotopes, however, are less common and often produced artificially in laboratories for research and industrial purposes.
What Is the Atomic Number of Sodium?
The atomic number of sodium is 11. In the periodic table, elements are arranged in order of increasing atomic number. Sodium is found in the third period and the first group of the periodic table. This means that all sodium atoms have 11 protons in their nuclei.
The atomic number is a fundamental property of an element, and it defines the element’s identity. Since each element has a unique number of protons in its nucleus, the atomic number serves as a characteristic identifier for that element.
- Industrial Applications: Sodium and its compounds have numerous industrial applications. Sodium hydroxide (NaOH), commonly known as caustic soda, is widely used in chemical processes, manufacturing, and cleaning agents. Sodium carbonate (Na2CO3), also known as soda ash, is used in glass production, water softening, and various chemical processes.
- Environmental Impact: The excessive intake of sodium through salt consumption can have health implications. Such as increased blood pressure and cardiovascular risks. Moreover, excessive sodium in soil or water bodies can have detrimental effects on plant growth and aquatic life. Managing sodium levels is, therefore, essential in both human health and environmental contexts.
Sodium is an alkali metal with chemical symbol Na, and it is highly reactive due to its single valence electron in the outermost electron shell. It is an essential element for various biological processes and is commonly found in salts, such as sodium chloride (table salt), as well as in many minerals and rocks. Its atomic number, 11, remains constant for all stable isotopes of sodium.