How & Why Is The Periodic Table Organized? The periodic table one of the most iconic and fundamental tools in chemistry, serving as a blueprint for understanding the properties and relationships of elements. It a systematic arrangement of chemical elements based on their atomic structure, enabling scientists and students alike to comprehend the behavior and characteristics of these building blocks of matter.
The organization of the periodic table may seem complex at first glance, but its underlying principles offer remarkable insights into the nature of elements and their significance in the universe. In this exploration, we will delve into the “How & Why” of the periodic table’s organization, uncovering the rationale behind its structure and the profound impact it has on modern science and technology.
How & Why Is The Periodic Table Organized
The periodic table a systematic arrangement of chemical elements, each represented by a unique symbol, organized based on their atomic number, electron configuration, and chemical properties. Dmitri Mendeleev, a Russian chemist, credited with the development of the first periodic table in the mid-19th century.
The organization of the periodic table allows scientists and researchers to understand the relationships and trends between elements more easily. The main driving force behind the organization the desire to group elements with similar chemical properties together, enabling a better understanding of the periodicity of their properties.
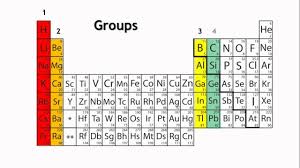
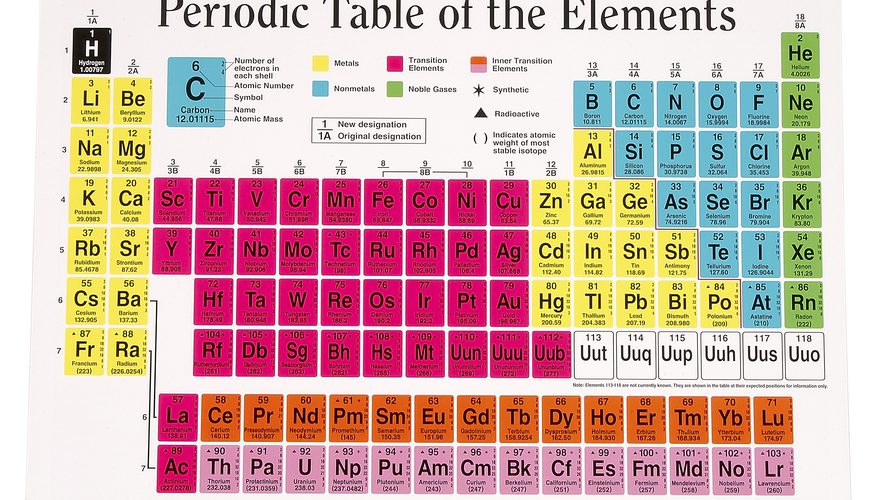
The periodic table organized in horizontal rows called periods and vertical columns known as groups. Elements in the same period share the same number of atomic shells, while elements in the same group have similar valence electron configurations. This arrangement allows for the identification of trends in properties as you move from left to right across a period and from top to bottom within a group. Elements located towards the left of the periodic table tend to metals, while those towards the right non-metals. The noble gases occupy the far-right column and known for their stable, unreactive nature.
In summary, the periodic table organized to bring out the relationships between elements and their properties. It helps scientists predict and understand the behavior of elements in chemical reactions and provides a solid foundation for studying the vast array of substances that make up our world. Learn More:- Periodic Tables with Names of Elements.
How Are The Elements Arranged In The Periodic Table
The arrangement of elements in the periodic table follows a specific order based on their atomic number, which represents the number of protons in an atom’s nucleus. When elements ordered by increasing atomic number, patterns in their properties emerge, forming the basis for the table’s structure. Each row in the periodic table corresponds to a period, and each column corresponds to a group.
The periodic table divided into several blocks based on electron configurations. The s-block elements, found in the first two groups on the left, have their outermost electrons in the s orbital. The p-block elements, in groups 13 to 18 on the right, have their outermost electrons in the p orbital. The d-block elements, located in the middle section, transition metals with their outermost electrons in the d orbital. Lastly, the f-block elements, located at the bottom, the inner transition metals with their outermost electrons in the f orbital.
Elements in the same group have similar chemical properties because they have the same number of valence electrons, leading to comparable reactivity. For example, alkali metals in Group 1 readily lose one electron to form a +1 cation, while halogens in Group 17 gain one electron to form a -1 anion. The transition metals, located in the d-block, share similar properties, such as forming colored compounds and displaying variable oxidation states.
In conclusion, the periodic table’s arrangement a result of organizing elements by their atomic numbers and electron configurations, leading to the emergence of patterns in their properties, making it a powerful tool for understanding and predicting chemical behavior.
Why Is The Periodic Table Arranged How It Is
The organization of the periodic table based on the periodic law, which states that the chemical and physical properties of elements periodic functions of their atomic numbers. Dmitri Mendeleev and Julius Lothar Meyer independently proposed the concept of the periodic table in the 1860s. Mendeleev’s version, published in 1869, widely recognized because he left gaps for yet-to-be-discovered elements and accurately predicted their properties.
The arrangement of elements in the periodic table vital for understanding their relationships and behavior. Elements in the same group share similar valence electron configurations, leading to comparable chemical properties. This similarity due to the fact that elements in the same group have the same number of valence electrons, which determines their reactivity and the type of chemical bonds they form.
The periodic table’s layout also highlights periodic trends in properties. Moving across a period from left to right, elements generally become smaller in size and have increasing ionization energy, electronegativity, and non-metallic character. Conversely, moving down a group, elements tend to increase in size, with lower ionization energy and higher metallic character.
The periodic table’s arrangement especially valuable for chemists and researchers as it helps them make predictions about unknown elements and understand the behavior of known elements in various chemical reactions. It serves as a foundation for the study of chemical bonding, molecular structure, and the behavior of substances under different conditions.
In conclusion, the periodic table’s organization a product of the periodic law, which governs the relationship between atomic numbers and element properties. The table’s layout allows scientists to discern patterns, classify elements, and make informed predictions, fostering a deeper understanding of the building blocks of the universe.