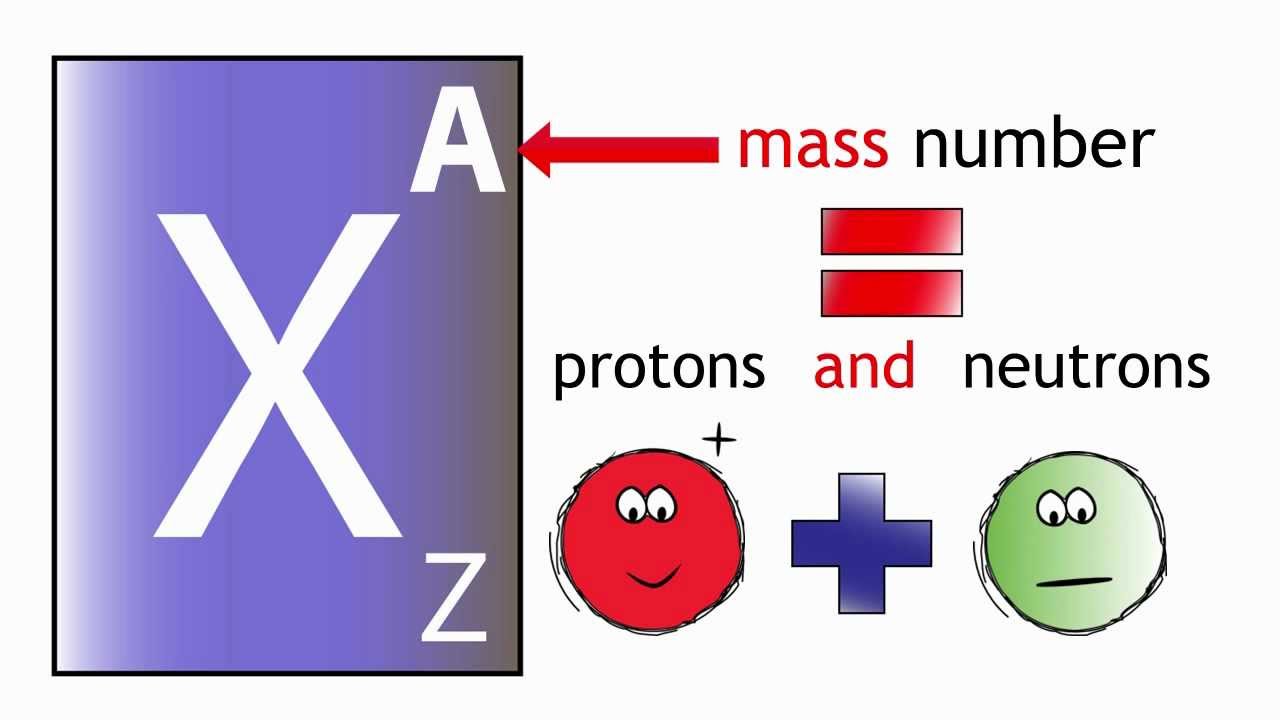
Element Atomic Number: The atomic number (symbol Z) is also called the proton number of a chemical element. It is the total number of protons that found in the nucleus of an atom. It is similar to the charge number of the nucleus. Through atomic number one can uniquely identify a chemical element. The atomic number is also equal to the number of electrons in, sodium, magnesium uncharged atom.
Element Atomic Number
The atomic number of an element is a fundamental property that distinguishes one element from another. It represents the number of protons found in the nucleus of an atom of that element. Each element has a unique atomic number, which determines its position in the periodic table and provides important information about its characteristics and behavior.
The atomic number is denoted by the symbol “Z” and is typically written as a subscript before the symbol of the element. For example, hydrogen has an atomic number of 1, so it is written as H₁. Carbon has an atomic number of 6, so it is written as C₆. The atomic number directly correlates to the number of protons in the nucleus because, in a neutral atom, the number of protons is equal to the number of electrons.
The atomic number is significant because it determines the identity of an element. Elements are defined by the number of protons in their nuclei, and even a slight change in the number of protons would result in a different element altogether. For example, if an atom has six protons, it will always be carbon, regardless of the number of neutrons or electrons it possesses. Check out other related posts:- Electronegativity Series, Seaborgium Valence Electrons.
The atomic number plays a crucial role in the organization of the periodic table. Elements are arranged in ascending order of their atomic numbers, which leads to their systematic grouping based on similar properties. The atomic number provides a clear sequence to the elements, allowing scientists to identify trends and patterns in their physical and chemical properties as they move across the periodic table.
Atomic Number of Elements from 1 to 30
In the picture below we are going to provide the atomic number of the element 1 to 30 that are hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon, aluminum, silicon, phosphorus, sulfur, chlorine, argon, potassium, calcium, scandium, Titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper and zinc.
- Hydrogen (H) – Atomic Number 1
- Helium (He) – Atomic Number 2
- Lithium (Li) – Atomic Number 3
- Beryllium (Be) – Atomic Number 4
- Boron (B) – Atomic Number 5
- Carbon (C) – Atomic Number 6
- Nitrogen (N) – Atomic Number 7
- Oxygen (O) – Atomic Number 8
- Fluorine (F) – Atomic Number 9
- Neon (Ne) – Atomic Number 10
- Sodium (Na) – Atomic Number 11
- Magnesium (Mg) – Atomic Number 12
- Aluminum (Al) – Atomic Number 13
- Silicon (Si) – Atomic Number 14
- Phosphorus (P) – Atomic Number 15
- Sulfur (S) – Atomic Number 16
- Chlorine (Cl) – Atomic Number 17
- Argon (Ar) – Atomic Number 18
- Potassium (K) – Atomic Number 19
- Calcium (Ca) – Atomic Number 20
- Scandium (Sc) – Atomic Number 21
- Titanium (Ti) – Atomic Number 22
- Vanadium (V) – Atomic Number 23
- Chromium (Cr) – Atomic Number 24
- Manganese (Mn) – Atomic Number 25
- Iron (Fe) – Atomic Number 26
- Cobalt (Co) – Atomic Number 27
- Nickel (Ni) – Atomic Number 28
- Copper (Cu) – Atomic Number 29
- Zinc (Zn) – Atomic Number 30
These elements span the first three periods of the periodic table and include a range of elements from gases (like hydrogen and helium) to metals (such as sodium, potassium, and zinc). Each element has a unique atomic number that corresponds to the number of protons in its nucleus, providing distinct properties and behaviors. Understanding the atomic numbers and elements within this range is foundational to studying chemistry and the periodic table.
Elements with Atomic Number and Mass
Elements in the periodic table are characterized by their atomic number and atomic mass. The atomic number, represented by the symbol “Z,” refers to the number of protons in the nucleus of an atom. The atomic mass, denoted by the symbol “A” or sometimes “m,” represents the average mass of an atom of an element, taking into account the combined mass of protons and neutrons in the nucleus.
Let’s take a look at a few examples of elements with their corresponding atomic numbers and atomic masses:
- Hydrogen (H):
- Atomic number (Z): 1
- Atomic mass (A): 1.00794 atomic mass units (amu)
- Carbon (C):
- Atomic number (Z): 6
- Atomic mass (A): 12.0107 amu
- Oxygen (O):
- Atomic number (Z): 8
- Atomic mass (A): 15.9994 amu
- Sodium (Na):
- Atomic number (Z): 11
- Atomic mass (A): 22.98976928 amu
- Iron (Fe):
- Atomic number (Z): 26
- Atomic mass (A): 55.845 amu
These examples represent a range of elements from different parts of the periodic table. It’s important to note that atomic masses are often not whole numbers due to the presence of isotopes, which are atoms of the same element with different numbers of neutrons. The atomic mass listed is usually an average of the different isotopes, taking into account their relative abundances.
How to Find an Element’s Atomic Number
Finding an element’s atomic number is relatively straightforward. Here’s how you can find the atomic number of an element:
- Consult the periodic table: The most reliable and efficient way to find an element’s atomic number is by referring to the periodic table. The periodic table is a tabular arrangement of elements, organized based on their atomic numbers. Each element is represented by a unique symbol and is accompanied by its atomic number.
- Locate the element: Look for the symbol of the element you are interested in on the periodic table. The symbols are usually one or two letters derived from the element’s name. For example, “H” represents hydrogen, “C” represents carbon, and “O” represents oxygen.
- Identify the atomic number: Once you locate the element on the periodic table, the atomic number typically listed above or below the element’s symbol. The atomic number is an integer that represents the number of protons in the nucleus of an atom of that element.
- Understand the significance: The atomic number is crucial because it uniquely identifies each element. It determines the element’s position in the periodic table and provides valuable information about its properties and behavior.
If you don’t have immediate access to a physical periodic table, numerous online resources and mobile applications provide interactive versions of the periodic table that can easily searched and accessed. These tools can quickly provide you with an element’s atomic number and other related information.
Atomic number = number of protons, and mass-number = number of protons + number of the neutron. You can check the atomic number of the element in the picture below.