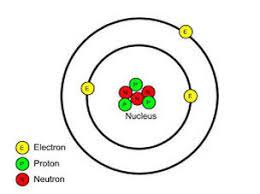
Electron Location In Atom: Electrons are the smallest and situated in the outermost shells or orbitals in an atom. It is negatively charged and cannot broken down further. It has a charge equal but opposite to that of protons, and due to this, they get attracted to protons. The number of electrons on the outermost shell is the basis of the chemical reaction of an element and help to determine the periodic table.
What are The Exact Electron Location In Atom?
The location of electrons within an atom is a fundamental concept in atomic physics, described by the electron cloud model. According to this model, electrons do not have fixed positions like planets in orbits around the nucleus. Instead, they exist in probability clouds, also known as orbitals. An orbital is a three-dimensional region around the nucleus where there is a high probability of finding an electron. The shape and size of the orbitals depend on the energy level and subshell in which the electrons reside.
The most commonly known orbitals the s, p, d, and f orbitals, each with distinct shapes and orientations. The s orbitals spherical and centered around the nucleus, while the p orbitals dumbbell-shaped and oriented along the x, y, and z axes. The d and f orbitals more complex in shape. It’s important to note that electron location is inherently uncertain due to the probabilistic nature of quantum mechanics.
The electron cloud model and the concept of probability orbitals provide a robust framework for understanding electron location in atoms, paving the way for advancements in fields such as chemistry, material science, and nanotechnology. Learn more about Electrons:- Electronegativity Series in Increasing Order, Electron Affinity Trend, Electron Affinity Equation.
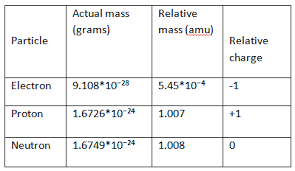
Electron Relative Mass
Electrons, subatomic particles with a negative charge, and they play a crucial role in the structure and behavior of atoms. When studying subatomic particles, it’s essential to consider their relative masses in comparison to other particles. The mass of an electron is significantly smaller than that of a proton or a neutron, the other two subatomic particles found in the nucleus of an atom.
The mass of electrons is much lesser than that of protons and neutrons, which have almost equal masses. The mass of an electron is about 0.000548597 a.m.u. or 9.1 x 10-31 kg, which is much less than the weight of protons.
To more precise, the relative mass of an electron is approximately 1/1836 of the mass of a proton or neutron. In other words, electrons nearly 1836 times lighter than protons and neutrons. This mass difference is one of the factors contributing to the vast difference in size between the nucleus and the electron cloud in an atom.
The relatively small mass of electrons allows them to move rapidly within the electron cloud and participate in various chemical reactions, bonding with other atoms to form molecules and compounds. This property of electrons is fundamental to the chemistry of life and the existence of matter as we know it.
What are Electrons and Where are They Located?
Electrons are subatomic particles that carry a negative electric charge. They fundamental constituents of atoms and critical to the behavior of matter. In the current understanding of atomic structure, electrons located outside the nucleus, occupying specific energy levels known as electron shells. Each shell can hold a limited number of electrons.
Electrons, together with protons and neutrons the sub-atomic particles which together form an atom. They negatively charged sub-atomic particles considered to elementary particles. Electrons located at the outer most shell or orbitals in an atom.
The first shell, closest to the nucleus, can hold up to two electrons, while subsequent shells can hold more. The second shell can hold up to eight electrons, and the third shell can hold up to 18 electrons. However, the actual arrangement of electrons in an atom can more complex due to the concept of subshells and the filling of orbitals based on energy levels.
Electrons distributed in these shells and subshells in a way that minimizes energy while adhering to certain rules, such as the Pauli exclusion principle and Hund’s rule. The exact location of an electron within an atom at any given moment is challenging to pinpoint due to the probabilistic nature of electron orbitals.
Can an Electron Be Found in an Exact Spot Within an Atom?
In the realm of classical physics, it would expected that electrons should have precise locations within an atom, much like planets in a solar system. However, quantum mechanics, the theory governing the behavior of subatomic particles, presents a different picture. According to the principles of quantum mechanics, electrons do not have exact positions within an atom.
Instead, they exist in regions of space called orbitals, which three-dimensional probability clouds where electrons likely to found. The shape and size of these orbitals determined by the quantum numbers associated with the electron’s energy level and angular momentum.
Heisenberg’s uncertainty principle further reinforces the idea that it is impossible to simultaneously know both the exact position and momentum of an electron. Attempting to locate an electron with pinpoint accuracy would disrupt its momentum. Making it impossible to predict its precise position within the atom.
Therefore, while we can describe the probabilities of finding an electron in a particular region around the nucleus, we cannot precisely determine its exact spot within an atom due to the inherent uncertainty of quantum mechanics.
It has been found that electrons show characteristics of both waves and particles, known as wave-particle duality. Werner Heisenberg conducted an experiment to locate the exact position of an electron. He used photon for his experiment. He came up with the Heisenberg Uncertainty Principle, according to which the exact location and position of an electron cannot determined.
Where are the Electrons Found in the Atom?
Electrons found in the atom’s electron cloud, which is the region surrounding the nucleus where electrons exist. The electron cloud is organized into discrete energy levels known as electron shells or energy shells. These shells labeled with quantum numbers (n=1, n=2, n=3, etc.), with the innermost shell (n=1) being the closest to the nucleus.
Electrons can found in the outermost shell or orbitals, surrounding the nucleus of an atom. Within each shell, there subshells, also known as energy sublevels, labeled as s, p, d, and f. The s subshell has a spherical shape, while the p subshell has a dumbbell shape. With three orientations along the x, y, and z axes. The d and f subshells have more complex shapes.
The distribution of electrons within the electron cloud follows the Aufbau principle, which states that electrons fill the lowest energy levels first before occupying higher energy levels. Moreover, the Pauli exclusion principle dictates that each orbital can hold a maximum of two electrons with opposite spins.
The exact arrangement of electrons within the electron cloud of an atom can represented using electron configurations. These configurations describe how many electrons in each subshell and energy level. Understanding the location of electrons in the atom is essential for comprehending the chemical properties. And reactivity of elements, as well as the formation of chemical bonds.